INTRODUCTION
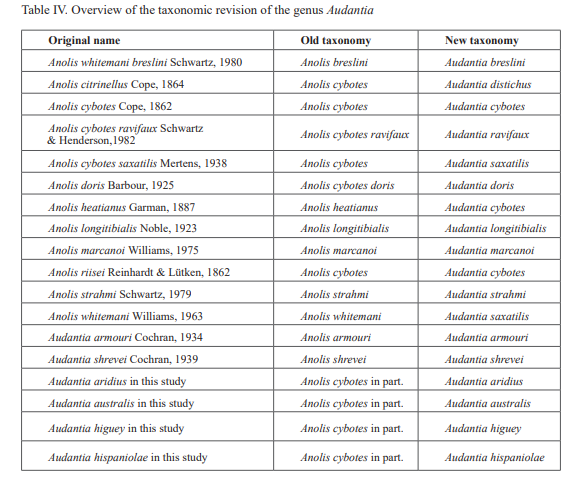
Anoles of the family Dactyloidae are a prominent faunal component on the major Antillean island of Hispaniola. Indeed, with 54 species, anoles form one of the most species-rich groups of amphibians and reptiles (total 261 species) on this island (Hedges, 2018). One group of anoles endemic to Hispaniola is the genus Audantia Cochran, 1934, formerly the Anolis cybotes series or subseries (Williams, 1976; Burnell & Hedges, 1990) and often informally referred to as the ‘cybotoids’ (Schwartz, 1979). The genus includes species that characteristically perch low to the ground (trunk/ground ecomorph) on bushes and trees and are among the most abundant anoles on the island, often occurring in degraded habitats (Henderson & Powell, 2009). The nine currently recognized species of Audantia (Schwartz, 1980; Schwartz & Henderson, 1982, 1991; Henderson & Powell, 2009; Nicholson et al., 2012, 2018) are A. armouri Cochran, 1934, A. breslini (Schwartz, 1980), A. cybotes (Cope, 1863), A. haetianus (Garman, 1887), A. longitibialis (Noble, 1923), A. marcanoi (Williams, 1975), A. shrevei Cochran, 1939, A. strahmi (Schwartz, 1979), and A. whitemani (Williams, 1963).
We are aware of the contentious debate between recognizing Anolis as a single genus and recognizing multiple genera (Nicholson et al., 2012; Nicholson et al., 2014; Poe, 2013; Poe et al., 2017; Nicholson et al., 2018). Here we follow Nicholson et al. (2018) and therefore recognize the genus Audantia Cochran, 1934 (sensu Nicholson et al., 2018) for the anole species in the cybotes series, while recognizing the criticisms of the multiple-genera taxonomy. The genus Audantia sensu Nicholson et al. (2018) corresponds to clade Audantia of Poe et al. (2017).
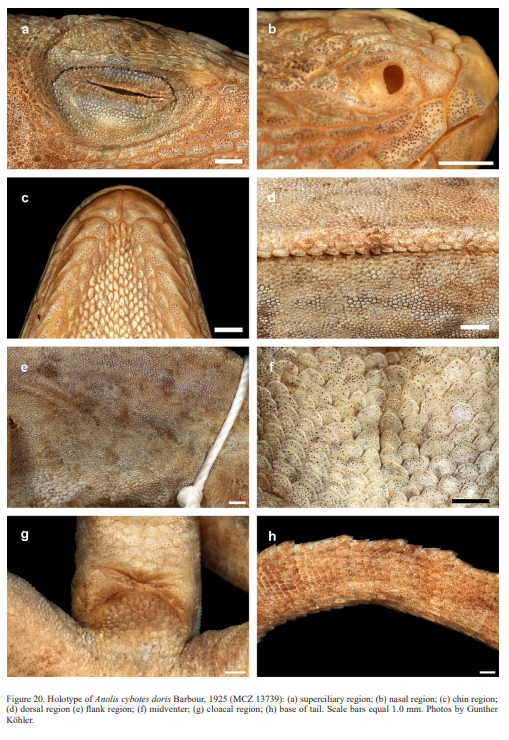
Cope (1863) introduced the new species Anolis cybotes based on five syntypes, ANSP 7604–05, MCZ 3619 (destroyed in 1939 according to Rosado, personal communication to Gunther Köhler, 25 April 2018) and MCZ 14346–47, that were collected in “Western Hayti; from near Jeremie”. Reinhardt & Lütken (1863) described Anolis riisei based on two syntypes (now NHMD R3796 and R3793) from “Haiti”. The new species Anolis citrinellus from “Hayti” was introduced by Cope (1864). Garman (1887) described Anolis haetianus based on three syntypes (MCZ 6191) from “Tiburon, Hayti”. According to Rosado, MCZ (personal communication to G. Köhler, 25 April, 2018), the three syntypes of A. haetianus are “a jumble of parts mixed together in pieces in a bottle”. In 1923, Noble “spent four days on Beata Island off the southwestern coast of the Dominican Republic” where he “made an effort to secure a representative collection”. Amongst the material collected was an adult male that became the holotype of his new species, Anolis longitibialis (now AMNH 24329), from “Beata Island, Dominican Republic” (Noble, 1923a). Cochran (1934; 1941) regarded this nominal taxon as a subspecies of A. cybotes. Other authors (e.g., Schwartz & Henderson, 1991; Nicholson et al., 2005) didn’t share this view and treated A. longitibialis as a distinct species. Barbour (1925) described the new species Anolis doris based on an adult male (now MCZ 13739) from “Gonave or Gonaive Island, off the west coast of Haiti” (= Île de la Gonâve, Haiti). Most authors have regarded this nominal taxon as a subspecies of A. cybotes (Cochran, 1941; Schwartz & Henderson, 1988).
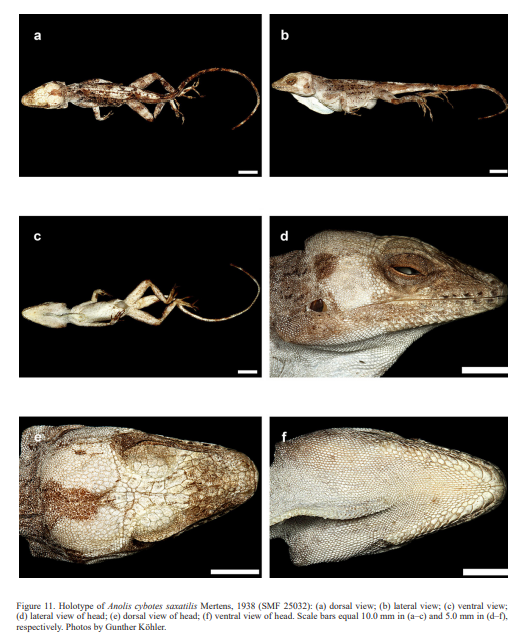
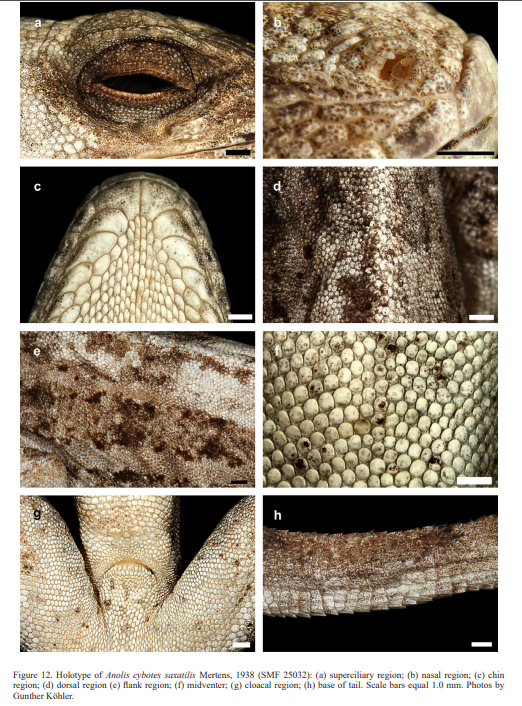
Cochran (1934) described the new species Audantia armouri based on an adult male (now MCZ 37523) “from Peak La Selle, Haiti”. This taxon was assigned to the genus Anolis by Etheridge (1960). In 1963, Williams recognized it as a subspecies of A. cybotes, but later treated it as a full species (Williams, 1976). This opinion was followed by Schwartz & Henderson (1991) and Nicholson et al. (2005). Anolis cybotes saxatilis was described by Mertens (1938) based on an adult male (now SMF 25032) from “Südlich von Fondo Negro, Gebiet des unteren Rio Yaque, Südwest-Santo Domingo” (= south of Fondo Negro, region of lower Rio Yaque, southwestern Dominican Republic).
Cochran (1941) placed the three nominal taxa A. riisei, A. citrinellus, and A. cybotes saxatilis in the synonymy of Anolis cybotes cybotes where they remained ever since (Uetz et al., 2019). Cochran (1939) introduced the new species Audantia shrevei based on an adult male (MCZ 44365) from “Valle Nuevo, in the Cordillera Central, southeast of Constanza, Dominican Republic, at 6000 to 8000 feet elevation,” which “resembles Audantia armouri Cochran, but has a large nuchal patch of enlarged keeled scales, has a darker and more uniform coloration, and attains a lager adult size”. This nominal taxon was listed as a synonym of A. armouri by most authors until now (Etheridge, 1960; Schwartz & Henderson, 1991). In 1963, Williams described Anolis whitemani based on an adult male (MCZ 60055) that was collected on a “road to Eaux Gaillees, Haiti”. He stated that it was similar to A. cybotes “but differing in squamation (…), and in color”. Williams (1975) introduced his new species Anolis marcanoi, which was named in honor of Professor Eugenio de Jesús Marcano, based on an adult male (holotype MCZ 131837) from “ca 5 km N La Horma, Peravia Province, Dominican Republic”. He stated that A. marcanoi is “quite distinct from A. cybotes in electrophoretic characters but nearly indistinguishable in squamation”.
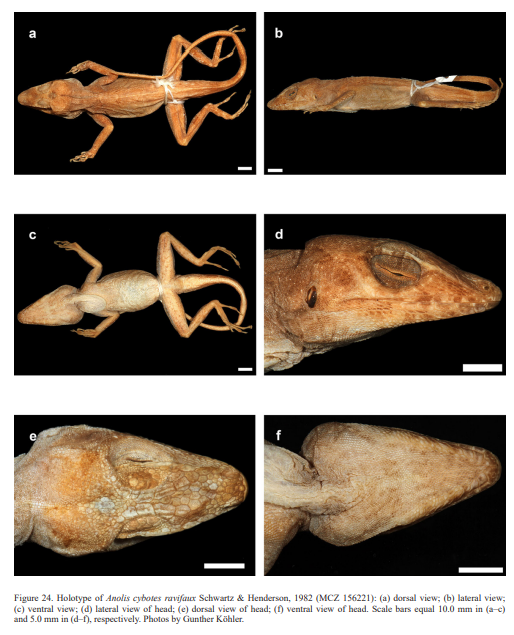
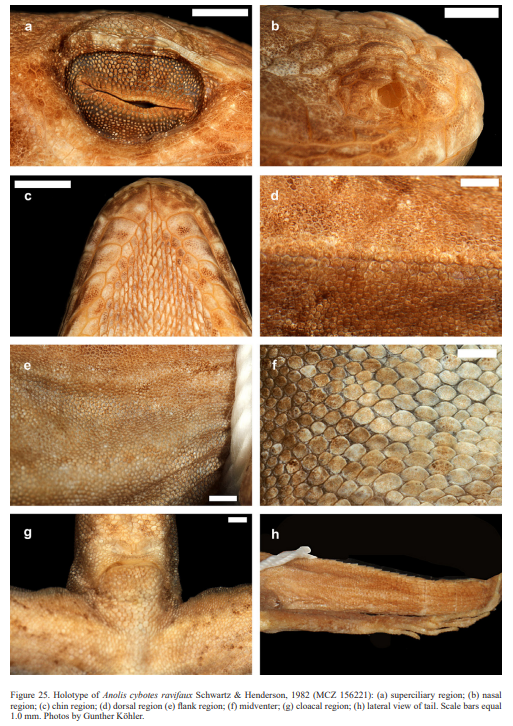
Schwartz (1979) described the new taxon A. longitibialis specuum based on an adult male (now MCZ 132370) from “17 km NW of Oviedo Nuevo, Pedernales Province, República, 183 m”. Schwartz (1979) also described the new species Anolis strahmi with two subspecies: Anolis strahmi strahmi based on an adult male (holotype MCZ 132371) from “3 km NE of El Aguacate, Independencia Province, 854 m, República Dominicana” and Anolis strahmi abditus based on an adult male (MCZ 146827) that was collected from a “dirt road to Las Mercedes, 2.9 km from intersection (= 5 km SE, 2.9 km N of Pedernales), Pedernales Province, República Dominicana”. Schwartz (1980) described two new taxa of anoles from Haiti, both as subspecies of A. whitemani: Anolis whitemani lapidosus (holotype MCZ 156206) from “Terre Sonnain, 1.6 km N Les Poteaux, 122 m, Département de l’Artibonite, Haiti” and Anolis whitemani breslini (holotype MCZ 156204) from “Môle St. Nicholas, Département du Nord-Ouest, Haiti”. The latter was elevated to full species status by Glor et al. (2003) who concluded that its “morphological distinctness, monophyly of sampled mtDNA haplotypes, deep divergence from all other sampled haplotypes (~10 % or more), and geographical isolation collectively support separate species status for A. breslini”. Schwartz & Henderson (1982) introduced the new subspecies Anolis cybotes ravifaux based on an adult male (holotype MCZ 156221) from “environs of Mano Juan, Isla Saona, República Dominicana”.
Despite an enormous activity in herpetological research in the Caribbean in general (see compilations e.g., in Schwartz & Henderson, 1991; Henderson & Powell, 2009) and with Caribbean anoles specifically (e.g., Losos, 2009 and references therein), the taxonomy of the cybotoid anoles of Hispaniola has not been addressed since the early 1980s. Our study of variation in genetic and morphological characters generated evidence for much more diversity than is reflected by the current taxonomy of this group of anoles, leading to this revision.
OBJECTIVES
- The objective of the present study is to revise the genus Audantia, using morphological and molecular data as lines of evidence, in order to define the morphological and geographical species boundaries in this group of lizards.
MATERIALS AND METHODS
For this study, we examined a total of 674 specimens of the genus Audantia (see Appendix 1). Abbreviations for museum collections follow Sabaj (2016) except for MNHNSD (Museo Nacional de Historia Natural “Prof. Eugenio de Jesús Marcano”, Santo Domingo, Dominican Republic). Coordinates and elevation were recorded using Garmin GPS receivers with built-in altimeters. All coordinates are in decimal degrees, WGS 1984 datum, and rounded to the appropriate decimal place given precision of the measurement. Prior to preservation of collected specimens in the field, we took color photographs of each individual’s extended dewlap. For this purpose, Gunther Köhler (GK) preferably utilized the standard forceps of genuine Swiss Army knives since their broad, flat apex prevents even thin-skinned dewlaps from damage and functions as an approximate scale (width = 3 mm in the models of both suppliers). Immediately after euthanasia via a pericardian injection of T61 (Intervet International, Unterschleißheim, Germany), relative hind limb length was determined by recording the point reached by the tip of the fourth toe when the extended hind limb was adpressed along the straightened specimen. Tissue samples were cut from the tip of the tail of selected individuals before they came in contact with formalin, stored in 98 % non-denatured ethanol.
Whenever possible, we everted the hemipenes of male specimens by injecting 70 % ethanol into the hemipenial pockets after manually pre-everting the hemipenes. Specimens were then preserved by injecting a solution of 5–10 mL absolute (i.e., 36 %) formalin in 1 L of 96 % ethanol into the body cavity and thighs, preferably also sprinkling everted hemipenes and extended dewlaps with this solution, and stored in 70 % ethanol. The collected specimens have been deposited in the collection of the Senckenberg Forschungsinstitut Frankfurt (SMF), Museo Nacional de Historia Natural “Prof. Eugenio de Jesús Marcano”, Santo Domingo, Dominican Republic (MNHNSD), and the Smithsonian Institution (USNM).
The capitalized colors and color codes (the latter in parentheses) are those of Köhler (2012). Terminology of markings used in color descriptions follow Köhler (2012). Nomenclature of scale characters follows that of Köhler (2014). Head length was measured from the tip of the snout to the anterior margin of the ear opening. Snout length was measured from the tip of the snout to the anterior border of the orbit. Head width was determined with the broad tips of the calipers aligned with the levels of posterior margin of eye and supralabial scales, respectively, with the calipers held in a vertical position relative to the head. Dorsal and ventral scales were counted at midbody along the midline. Tail height and width were measured at the point reached by the heel of the extended hind leg. Subdigital lamellae were counted on Phalanges II to IV of Toe IV of the hind limbs, and separately on distal phalanx. We considered the scale directly anterior to the circumnasal to be a prenasal.
Abbreviations used are AGD (axilla-groin distance), dorsAG (number of medial dorsal scales between levels of axilla and groin), dorsHL (number of medial dorsal scales in one head length), HDT (horizontal diameter of tail), HL (head length), HW (head width), IFL (infralabials), IP (interparietal plate), SAM (scales around midbody), ShL (shank length), SL (snout length), SO (subocular scales), SPL (supralabial scales), SS (supraorbital semicircles), SVL (snout-vent length), TL (tail length), VDT (vertical diameter of tail), ventrAG (number of medial ventral scales between levels of axilla and groin), and ventrHL (number of medial ventral scales in one head length). Synonymies in the species accounts are restricted to the major checklists of the Caribbean herpetofauna, regional treatments, and relevant taxonomic works.
Discriminant function analysis (DFA) was used to evaluate the phenetic distinctness of a priori groups (i.e., genetic clusters). The DFAs were created with the aid of the computer programm STATISTICA 6 (StatSoft, Inc. 2003). Discriminant scores (DS) were calculated by multiplying selected standardized variables (raw variables minus their associated mean value and divided by their associated standard deviation) by their associated unstandardized canonical coefficients. Each specimen was then plotted along the axes providing maximal separation of the a priori groups.
It is known that Hispaniola is a composite of two separate paleo-islands that collided about 10 million years ago (Hedges, 1996). Herein we refer to the southern and northern regions as the South Island and North Island, respectively, following Mertens (1939) and Schwartz (1978). Today they are connected by dry land that is below sea level, the Valle de Neiba (Dominican Republic) and Cul de Sac (Haiti).
As lines of evidence for species delimitation, we apply a phenotypic criterion (external morphology: coloration, morphometrics, and pholidosis, all structures coded by nuclear genes) and a criterion for reproductive isolation (genetic distinctness of the mitochondrial cytochrome B and ND2 genes). Genomic DNA was extracted from tissue using the DNeasy Blood and Tissue kit (Qiagen, Massachusetts, USA). For degraded samples and those for which sufficient yield was not obtained with the Qiagen kit, phenol chloroform extractions were completed. PCR amplification was performed under standard reaction conditions as outlined elsewhere (Hedges et al., 2008).
For this study, we have added a total of 267 new cytB sequences and 280 ND2 sequences (Appendix 2), with 142 ND2 sequences obtained from Glor et al. (2003). It should be noted that S.Blair Hedges (SBH) provided tissues for use in Glor et al. (2003) but they were reported incorrectly in that study as “USNM” catalog numbers when instead they were field and laboratory numbers used by SBH. They are represented in this tree with their correct “SBH” field numbers (see Appendix 2). The primers used to obtain these sequences were as follows: cytB – cytBL3
5’ ATACAYTACACAGCRGAYAT 3’ and cytBH3 5’TGGGTGTTCKACTGGTTGTCC3’; ND2 (from Macey et al 1997) – L4437 5’AAGCTTTCGGGCCCATACC3’ and H4980 5’ATTTTTCGTAGTTGGGTTTGRTT3’. Sequences from 426 ingroup and three outgroup taxa were analyzed resulting in a total of 1691 aligned sites. Gene alignments were performed independently in Geneious 11.0.4 (https://www.geneious.com) using MAFFT 7.388 with default parameters (Katoh & Standley, 2013). Protein translations were reviewed to ensure correct alignment with respect to reading frames and individual gene trees were constructed as an additional check on data quality. Best-fit model selection for each gene was performed in MEGA X (Kumar et al., 2018) and a maximum likelihood (ML) analysis was completed using RAxML 8.2.11 (Stamatakis, 2014), in Geneious 11.0.4. Evolutionary rates and base frequencies were estimated independently per gene, using the evolutionary model GTR + I + Γ. Gaps were treated as missing data. All parameters for the ML analyses were estimated by the program during the run. Branch support in the trees was provided by standard bootstrap analysis (2,000 replicates).
RESULTS
Taxonomy of anoles of the genus Audantia
The species of the genus Audantia are readily differentiated from all other Hispaniolan anoles (family Dactyloidae) by the combination of having (1) a very large head in adult males (ratio head length/SVL >0.3); (2) predominantly grayish-brown overall coloration in life; (3) usually a double row of slightly to greatly enlarged vetrebral scales, not forming a serrated crest (4) moderately long hind legs (fourth toe of adpressed hind leg reaching to a point between of posterior margin of eye and tip of snout); (5) the ventral scales at midbody smooth or keeled; (6) 24–35 subdigital lamellae on Phalanges II–IV of Toe IV of hind limbs; (7) male dewlap dirty white, greenish, yellowish, or orange; (8) males with a pair of distinctly enlarged postcloacal scales.
The analysis of our molecular data revealed 14 distinct genetic clusters among the specimens of the genus Audantia we studied (Fig. 1). We take this high level of genetic differentiation among these clusters (Table I) as evidence for lack of gene flow. Also, we documented morphological differences among these 14 genetic clusters, both in single characters of external morphology (Table II) as well as in multivariate statistical analyses.
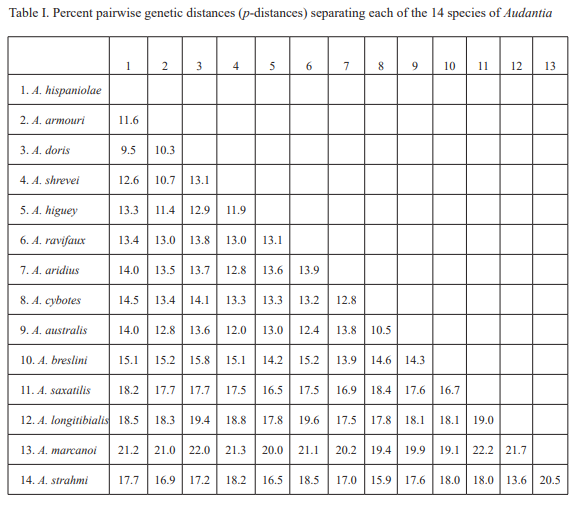
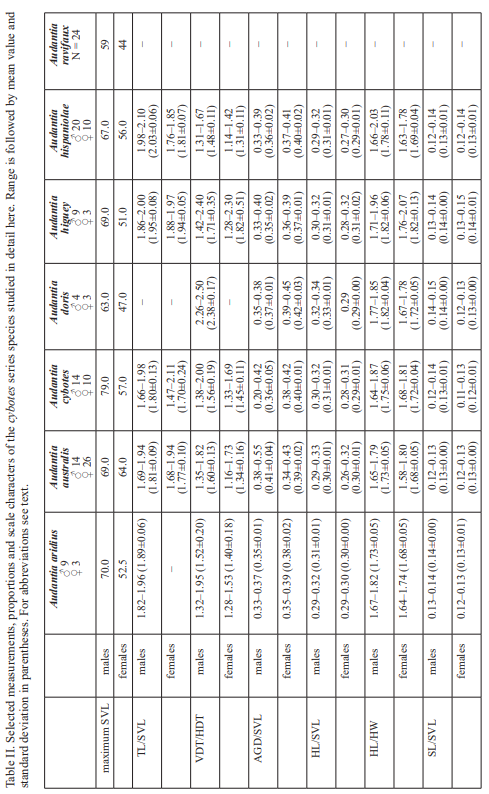
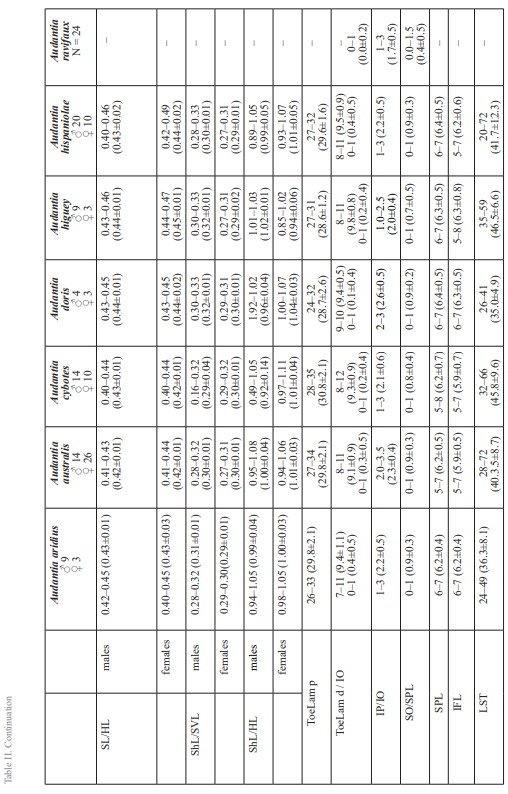
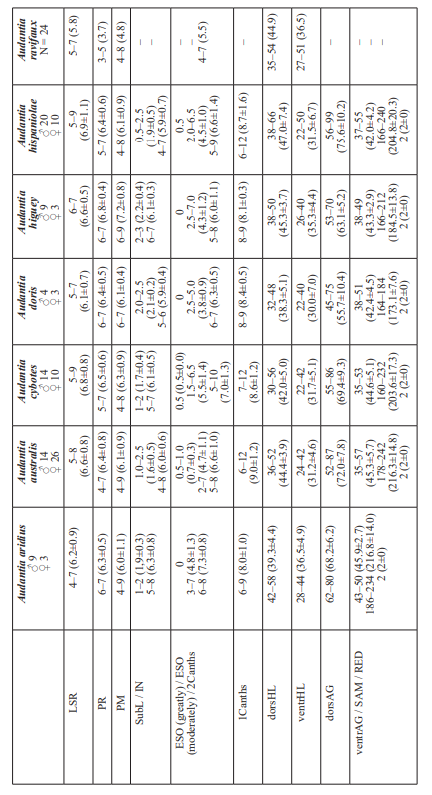
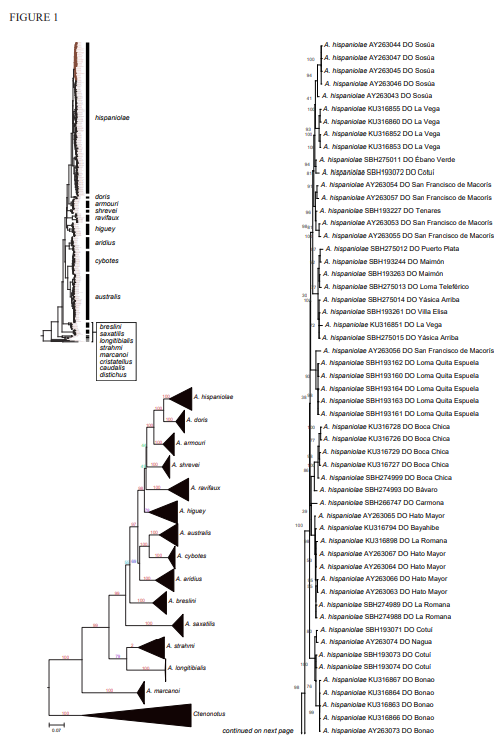
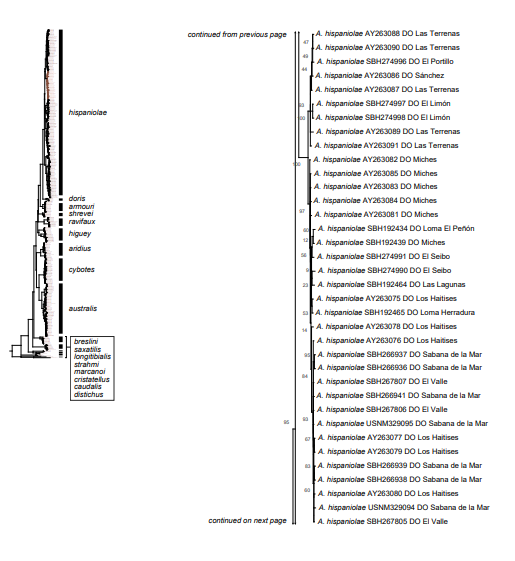
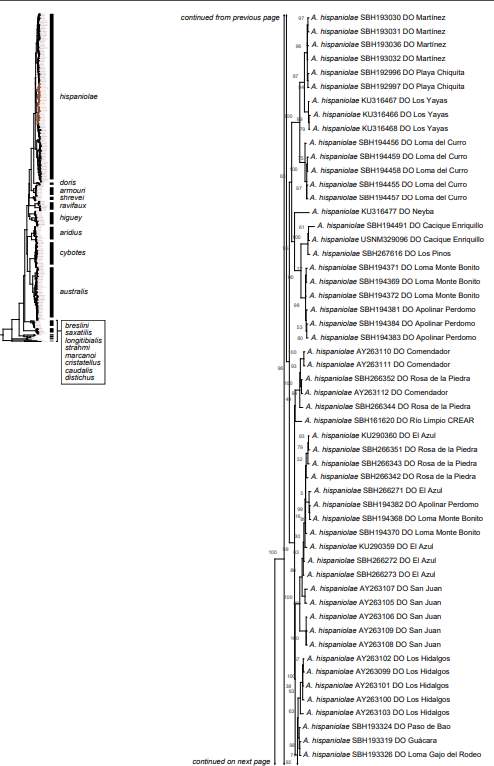
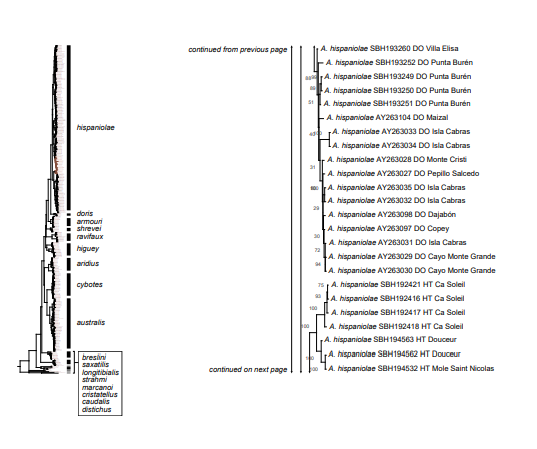
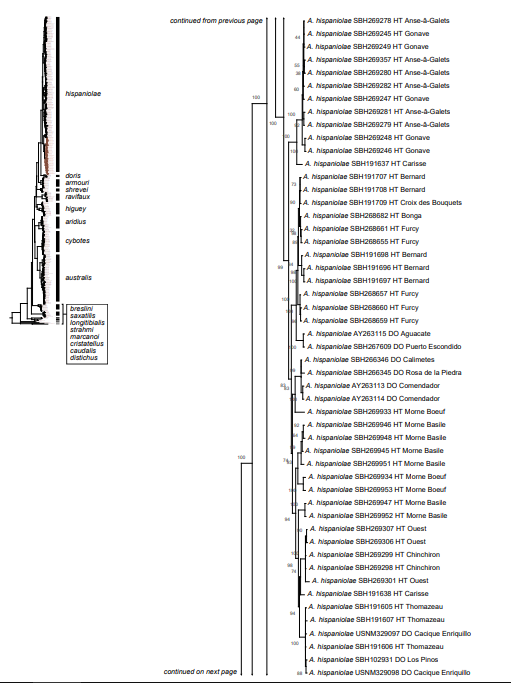
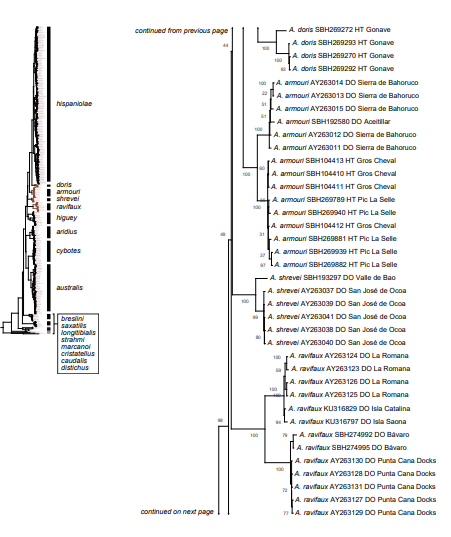
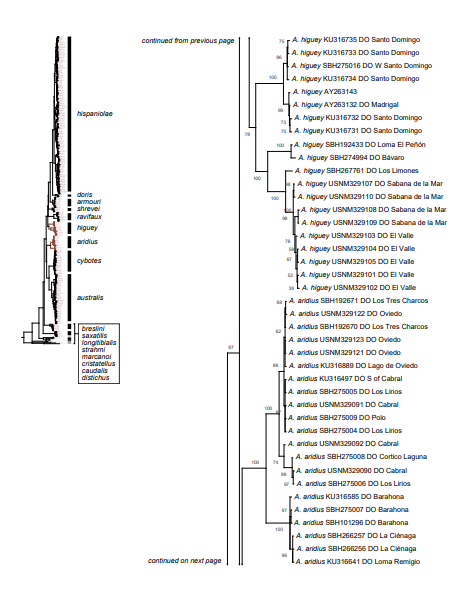

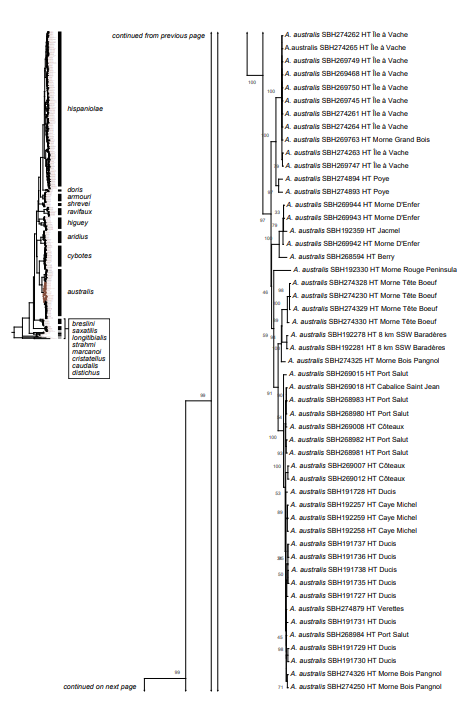
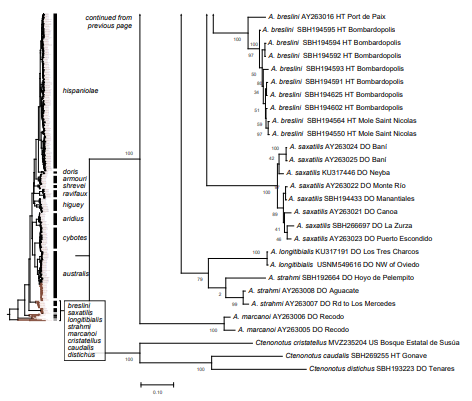
Figure 1. Phylogenetic tree of specimens of the genus Audantia from a maximum-likelihood analysis of DNA sequences of two mitochondrial genes: cytochrome b and ND2. A scale bar is indicated. The numbers at nodes are bootstrap values. The tree is rooted with the species Ctenonotus caudalis, C. cristatellus, and C. distichus. A locator tree is shown on each page identifying the clade or clades (red) that are displayed on the same page. For ease of viewing, a species-level tree is shown as an inset in the lower left corner of the first page, with same topology, branch lengths, and support values as in the full tree. Black triangles are proportional to the number of individuals within each species in the full tree.
Figures 2–7 show the results of discriminant function analyses (DFA). The best discriminating characters were relative size of body scales, number of loreal scales, as well as the ratios shank/SVL and AGD/SVL.
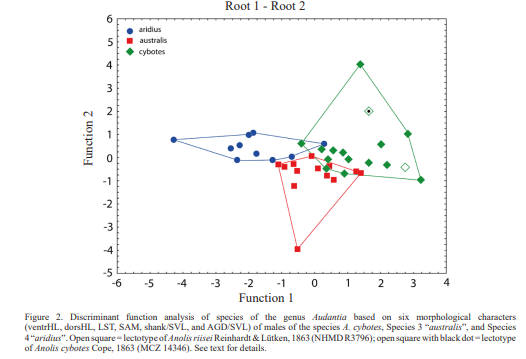
Figure 2 shows the results of an analysis based on six morphological characters (ventrHL, dorsHL, LST, SAM, shank/SVL, and AGD/SVL) of males of the species Audantia cybotes, Species 3 “australis”, and Species 4 “aridius”. The first and second discriminant functions correctly classified 80.0 % of the specimens of A. cybotes, 84.6 % of Species 3 “australis”, and 80.0 % of Species 4 “aridius”. The first discriminant function is DS = 0.048747 [ventrHL] -0.901191 [dorsHL] + 0.754006 [LST] -0.436889 [SAM] -0.381972 [shank/SVL] + 0.052856 [AGD/SVL]. The second discriminant function is DS = -0.138031 [ventrHL] + 0.183618 [dorsHL] + 0.064224 [LST] -0.168316 [SAM] -0.317627 [shank/SVL] -0.948841 [AGD/SVL]. The polygons of all three species hardly overlap.
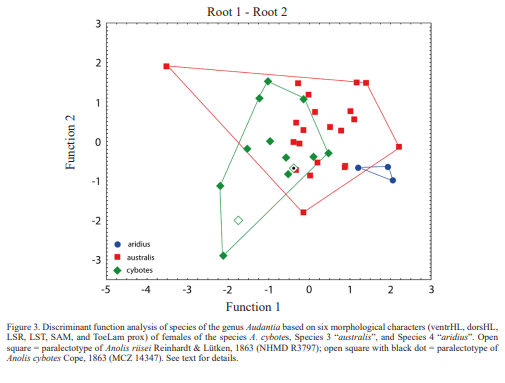
Figure 3 shows the results of an analysis based on six morphological characters (ventrHL, dorsHL, LSR, LST, SAM, and ToeLam prox) of females of the species A. cybotes, Species 3 “australis”, and Species 4 “aridius”. The first and second discriminant functions correctly classified 41.7% of the specimens of A. cybotes, 90.5 % of Species 3 “australis”, and 33.3 % of Species 4 “aridius”. The first discriminant function is DS = 0.222672 [ventrHL] + 0.723711 [dorsHL] -0.552333 [LST] -0.175056 [SAM] + 0.300687 [shank/SVL] -0.406005
[A-GD/SVL]. The second discriminant function is DS = -0.265324 [ventrHL] + 0.275709 [dorsHL] -0.540854 [LST] + 0.565826 [SAM] + 0.425727 [shank/SVL] -0.168236 [A-GD/SVL]. The polygone of Species 4 “aridius” does slightly overlap with the polygone of A. cybotes, whereas A. cybotes’ and Species 3 “australis”’ polygons have a high degree of overlap.
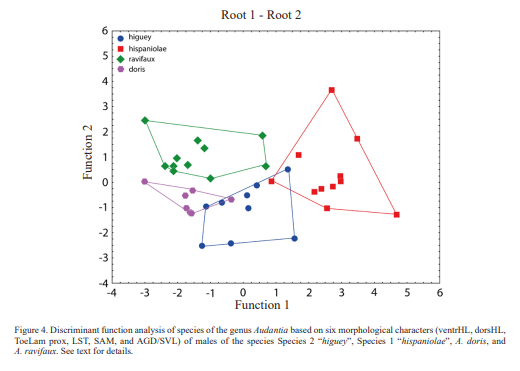
The results of an analysis based on six morphological characters (ventrHL, dorsHL, ToeLam prox, LST, SAM, and AGD/SVL) of males of the species Species 2 “higuey”, Species 1 “hispaniolae”, A. doris, and A. ravifaux are shown in Figure 4. The first and second discriminant functions correctly classified 77.8 % of the specimens of Species 2 “higuey” and 81.8 % of Species 1 “hispaniolae”, 71.4 % of A. doris, and 91.7 % of A. ravifaux. The first discriminant function is DS = -0.390318 [ventrHL] -0.054148 [dorsHL] + 0.837806 [SAM] + 0.448478 [LST] + 0.183539 [AGD/SVL]. The second discriminant function is DS = 0.731362 [ventrHL] + 0.296961 [dorsHL] + 0.523924 [SAM] -0.946471 [LST] + 0.140161 [AGD/SVL]. The polygone of A. ravifaux does not overlap with any of the other polygons, whereas whereas the polygons of the remaining three species slightly overlap with each other.
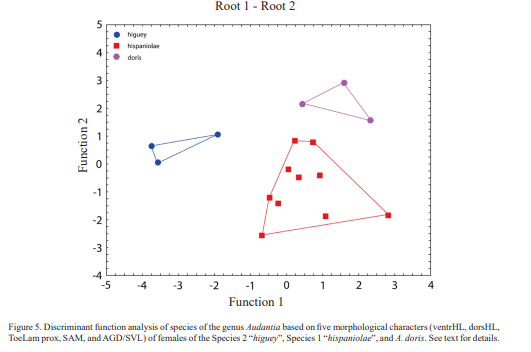
Figure 5 shows the results of an analysis based on five morphological characters (ventrHL, dorsHL, ToeLam prox, SAM, and AGD/SVL) of females of the Species 2 “higuey”, Species 1 “hispaniolae”, and A. doris. The first and second discriminant functions correctly classified 100.0 % of all three specimens. The first discriminant function is DS = -0.618265 [ventrHL] -0.331903 [dorsHL] -0.952501 [LST] + 1.533832 [SAM] + 0.966015 [AGD/SVL]. The second discriminant function is DS = -0.17553 [ventrHL] -1.15786 [dorsHL] -1.32790 [LST] -0.61151 [SAM] + 0.00630 [AGD/SVL]. All three polygons do not overlap.
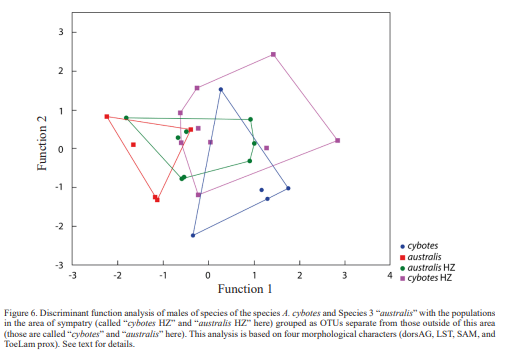
To further evaluate the patterns of morphological variation in the genetic clusters that occur sympatrically at several localities, we placed the genetically defined specimens in the area of sympatry in OTUs separate from those outside of this area. In the case of A. cybotes and Species 3 “australis”, this resulted in completely separated polygons for the allopatric OTUs of these taxa, whereas the polygons of the OTUs in the area of sympatry strongly overlap, with only 33.3–66.6 % of the OTUs in the area of sympatry being classified correctly (Figs. 6 and 7). For the males, the first discriminant function is DS = -0.16628 [ToeLam prox] + 0.80227 [LST] -0.73931 [SAM] -0.56962 [dorsAG]. The second discriminant function is DS = 0.34617 [ToeLam prox] + 0.13400 [LST] -0.62410 [SAM] + 1.00712 [dorsAG]. For the females, the first discriminant function is DS = 0.06983 [ToeLam prox] -0.88473 [ToeLam dist] + 0.07918 [LST] + 0.42101 [IO] -0.41888 [SAM] + 0.09967 [dorsAG]. The second discriminant function is DS = -0.37742 [ToeLam prox] + 0.42339 [ToeLam dist] -0.59342 [LST] + 0.66923 [IO] + 0.22095 [SAM] + 0.13491 [dorsAG].
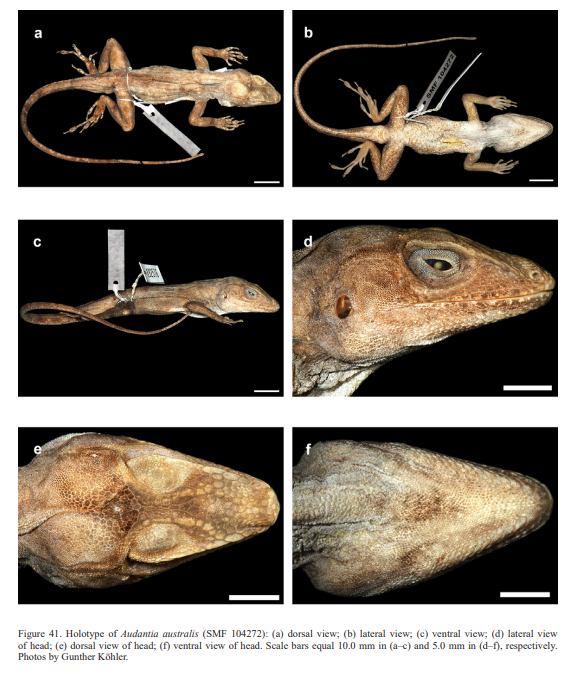
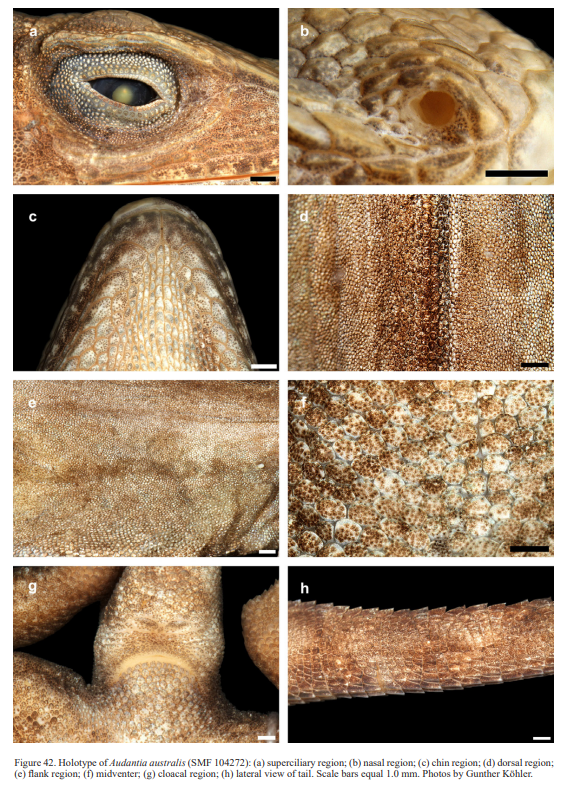
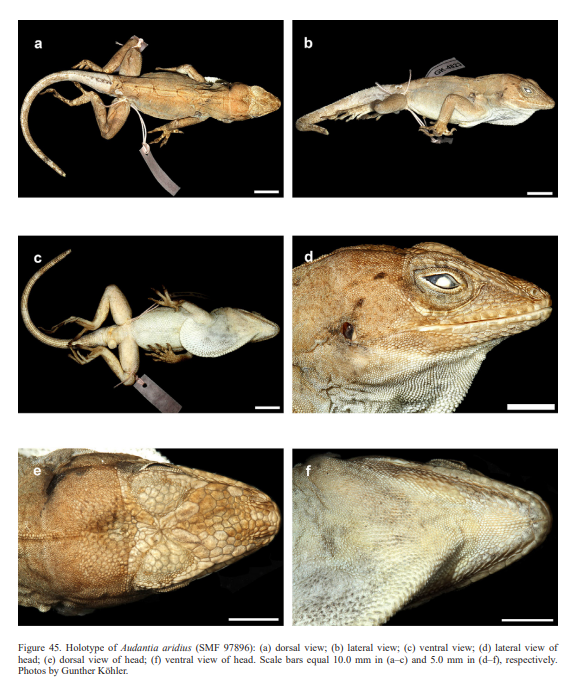
In conclusion, we recognize these 14 clusters as species level units. The majority of the species level units we recognize can be readily assigned to a nominal taxon based on the geographic provenance of the respective type material (i.e., Audantia armouri, A. breslini, A. doris, A. longitibialis, A. marcanoi, A. ravifaux, A. shrevei, A. strahmi, A. whitemani). The type locality of Anolis cybotes Cope, 1863 is “Western Hayti; from near Jeremie”.

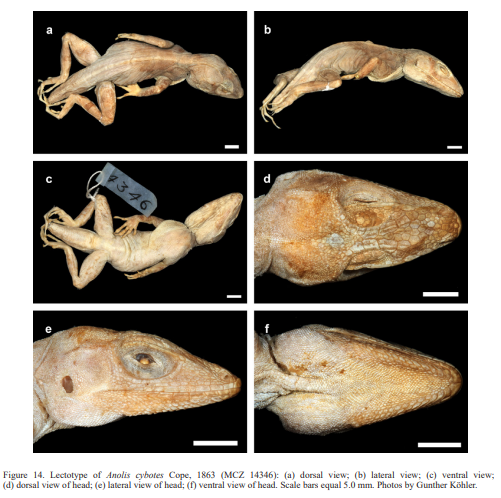
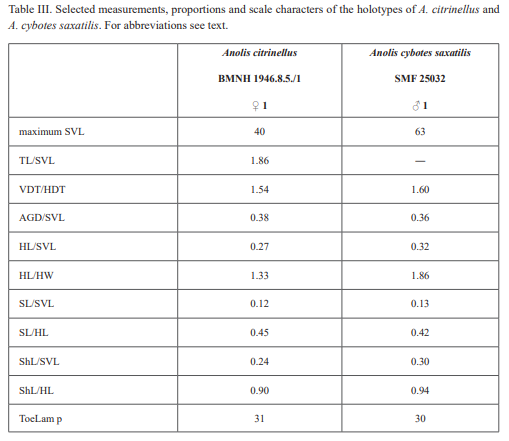
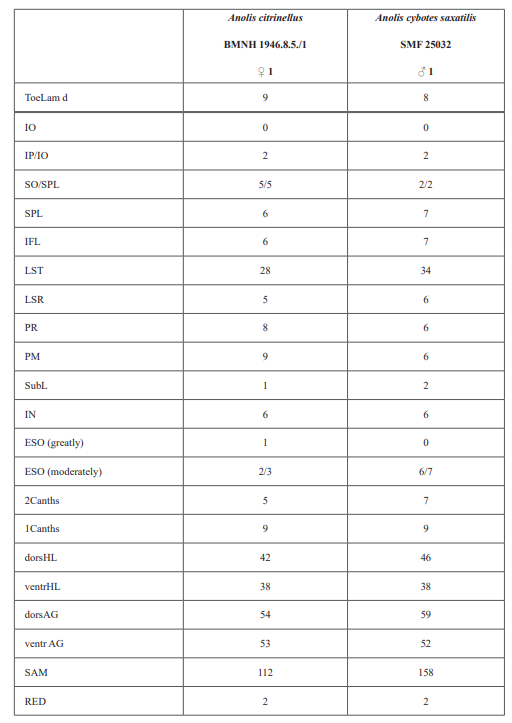
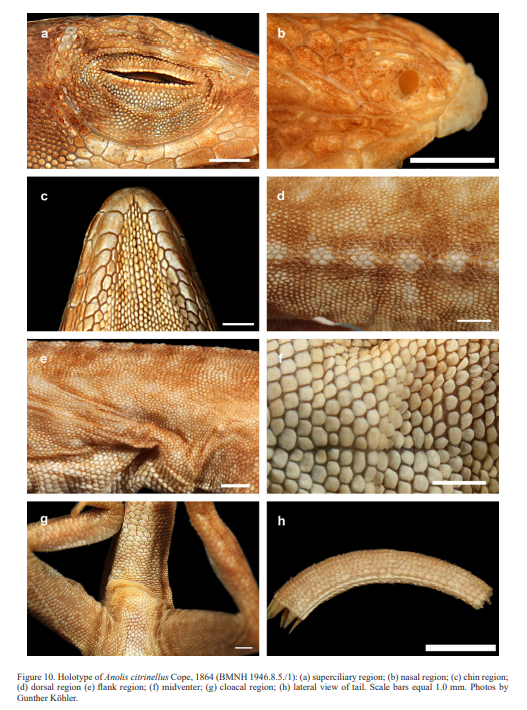
Therefore, we assign the genetic clade that contains specimens from the extreme western portion of the Tiburón Peninsula, Haiti, to A. cybotes sensu stricto. Also, we here designate as the lectotype of Anolis cybotes Cope, 1863 MCZ 14346, an adult male. Given its type locality and the poor state of conservation of its type material (see introduction and Fig. 8), and the genetic evidence, we assign the nominal taxon Anolis haetianus Garman, 1887 to the synonymy of A. cybotes Cope, 1863. Our data support the recognition of the nominal taxa Anolis cybotes ravifaux Schwartz & Henderson, 1982 and Anolis doris Barbour, 1925, respectively, as distinct species. Finally, we examined the holotype of Anolis citrinellus Cope, 1864 (BMNH 1946.8.5./1) and were surprised to learn that this specimen is not a species of the genus Audantia but rather represents an adult female of Ctenonotus distichus (new synonymy; Figs. 9–10; Table III). Furthermore, the holotype of Anolis cybotes saxatilis Mertens, 1938 (Figs. 11–12; Table III) is actually a specimen of the biological species currently referred to as A. whitemani, not of A. cybotes, as characterized by having dark brown crossbands on neck and anterior dorsum, keeled ventral scales; homogeneously distributed, widely spaced small gorgetals with more skin covered than uncovered by scales; no dark gular streaks; and a double row of weakly enlarged, but distinct vertebral scales. Therefore, we consider Anolis cybotes saxatilis Mertens, 1938 and Anolis whitemani Williams, 1963 to be conspecific and place the latter name into the synonymy of the former. Thus, in this work we use the name A. saxatilis for the species formerly referred to as A. whitemani.
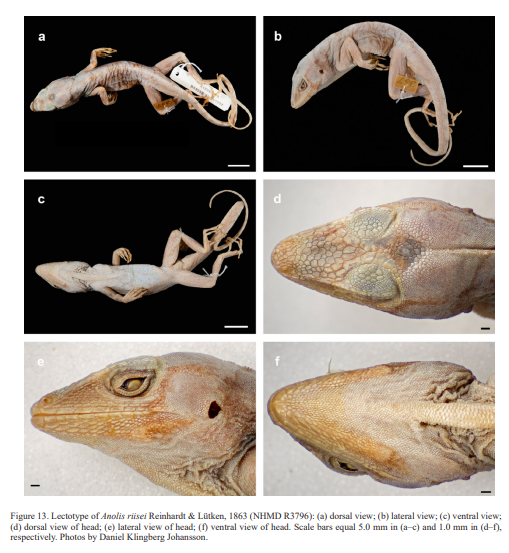
A somewhat complicated issue is the taxonomic identity of Anolis riisei Reinhardt & Lütken, 1863. This taxon was descibed based on two syntypes, NHMD R3796 (adult male) and R3793 (adult female) from “Haiti,” a term that was used for the whole island of Hispaniola at that time. Herewith we designate the male syntype, NHMD R3796 (Fig. 13), as the lectotype of Anolis riisei Reinhardt & Lütken, 1863 and in the following provide a redescription of this specimen.
NHMD R3796, adult male, as indicated by well-developed dewlap, and presence of a pair of greatly enlarged postcloacal scales; SVL 65.0 mm; tail length 113.0 mm (complete); tail distinctly compressed in cross section, tail height 4.0 mm and width 1.9 mm; axilla to groin distance 25.3 mm; head length 19.0 mm, head length/SVL ratio 0.29; snout length 8.3 mm; head width 10.6 mm; longest toe of adpressed hind limb reaching to level of anterior margin of eye; shank length 18.0 mm, shank length/head length ratio 0.95; longest finger of extended forelimb reaching to 5 mm past snout; longest finger of adpressed forelimb reaching to 2 mm past anterior insertion of hind limbs. Dorsal head scales mostly keeled, some rugose or smooth, especially in frontal and parietal regions; 7 postrostrals; 7 scales between nasals; 1 elongate prenasal scale on each side, distinct from circumnasal and in contact with both rostral and first supralabial; circumnasal separated from first supralabial by two scales; scales in deep prefrontal depression mostly slightly keeled; supraorbital semicircles well-developed, broadly in contact medially; supraorbital disc composed of 4 to 5 moderately enlarged, keeled scales arranged in three to four rows; circumorbital row complete, therefore, enlarged supraorbital scales separated from supraorbital semicircles; a very large elongated superciliary, followed posteriorly by a much smaller, overlapping one and by several small, keeled scales; about five rows of small keeled scales extending between enlarged supraorbitals and large superciliary; a parietal depression present; interparietal scale well-developed, 2.3 x 1.5 mm (length x width), surrounded by scales of moderate size; 2 scales present between interparietal and supraorbital semicircles; canthal ridge distinct, composed of 3 large and 2 small anterior canthal scales; 8 scales present between second canthals; 9 scales present between posterior canthals; 46 (right) – 49 (left) mostly keeled loreal scales in a maximum of 9 horizontal rows; 7 keeled subocular scales arranged in a single row; 6 supralabials to level below center of eye; suboculars separated from supralabials by a complete scale row; ear opening 1.3 x 1.9 mm (length x height); mental distinctly wider than long, almost completely divided medially, bordered posteriorly by 6 postmentals, outer ones much larger than median ones; 6 infralabials to level below center of eye; sublabials greatly enlarged (< four times the size of medial postmental scales), one in contact with infralabials; scales in sublabial row much larger than scales medially adjacent to this row; granular scales present on chin and throat; dewlap large, extending from level below anterior margin of eye onto chest; a nuchal crest and a dorsal ridge present; dorsum of body with keeled, granular scales; 2 medial rows distinctly enlarged, mostly usually less than twice the size of adjacent scales; largest dorsal scales about 0.40 x 0.30 mm (length x width); about 46 medial dorsal scales in one head length; about 70 medial dorsal scales between levels of axilla and groin; lateral scales keeled, granular and more or less homogeneous in size, average size 0.10 mm in diameter; ventrals at midbody smooth, flat, almost cycloid, imbricate, about 0.45 x 0.75 mm (length x width); about 32 medial ventral scales in one head length; about 46 medial ventral scales between levels of axilla and groin; 190 scales around midbody; all caudal scales keeled; middorsal caudal scales distinctly enlarged, forming a low crest; lateral caudal scales without whorls of enlarged scales, although an indistinct division in segments is discernible; a pair of greatly enlarged postcloacal scales present, about 1.1 mm wide; no tube-like axillary pocket present; scales on dorsal surface of upper forelimb strongly keeled, mucronate, imbricate; scales on anterior surface of thigh keeled, mucronate, imbricate; digital pads dilated, dilated pad three times the width of non-dilated distal phalanx; distal phalanx narrower than and raised from dilated pad; 32 lamellae under Phalanges II–IV of Toe IV of hind limbs; 10 scales under distal phalanx of Toe IV of hind limbs.
In external morphology, the A. riisei lectotype NHMD R3796 agrees well with the specimens from the western portion of the Tiburón Peninsula, Haiti, that we have assigned to A. cybotes. This observation is supported in a multivariate analysis, where the lectotype of A. riisei is placed in the center of the A. cybotes morphospace. Thus, we consider the primary type specimens of Anolis cybotes Cope, 1863 and Anolis riisei Reinhardt & Lütken, 1863 to belong to the same biological species.
The publication “Bidrag til det vestindiske Øriges og navnligen de dansk-vestindiske Øers Herpetologie” has often been cited as “Reinhard & Lütken, 1863”. However, the publication from 1863, printed in Copenhagen by “Bianco Lunos Bogtrykkeri ved F.S. Muhle,” appears to be a reproduction of a work that was originally communicated on 14 February 1862 in the journal “Videnskabelige Meddelelser fra den naturhistoriske Forening i Kjöbenhavn”. In the former publication the original date of publication (14 February 1862) is in fact mentioned on page 1, which corresponds to page 153 in the latter paper. Hence, the content of both publications is identical (with the exception of the first page, which includes additional information on journal number etc. in the original paper) but the page numbering differs.
Interestingly, something similar appears to be the case with the journal containing the Cope paper describing Anolis cybotes. That journal was also printed in 1863. At the bottom of the cover page of the journal it says “Philadelphia: Printed for the Academy. 1863”. The journal contains papers that were “presented for publication” (i.e., not published) between January and December 1862. The Cope paper is first mentioned on page 160, where it says “April 22d, 1862. Forty members present. The following papers were presented for publication: Contributions to Neotropical Saurology, by E. D. Cope” The Cope paper begins on page 176 and on the bottom of that page is printed “[April,” and on page 177 “1862.]”. Thus, although both works (i.e., that of Cope and that of Reinhard & Lütken) have been presented in some way in 1862, both seem to have been printed in 1863. There is no indication that either article was released as a single individual paper prior to the publication of the entire volume in 1863. We have no information as to the exact date when these two articles were actually published in 1863, and therefore are unable to determine which one has been published first. Given the long usage of the name Anolis cybotes Cope, 1863 for a well-known biological species, we continue to use this name and maintain Anolis riisei Reinhardt & Lütken, 1863 in the synonymy of Anolis cybotes Cope, 1863, a species name we restrict to the populations inhabiting the western portion of the Tiburón Peninsula, Haiti.
In conclusion, we recognize 14 species of anoles in the genus Audantia (i.e., A. armouri, A. breslini, A. cybotes, A. doris, A. longitibialis, A. marcanoi, A. ravifaux, A. saxatilis, A. shrevei, A. strahmi, as well as four undescribed species). In the standard characters of external morphology, these 14 species are not easily differentiated (Table II). However, subtle differences in body and dewlap scalation, morphometrics, and dewlap coloration among these species are useful to differentiate them. No names are available for four of our species level units, and therefore we describe each of them as a new species below.
In the following, we provide species accounts for Audantia cybotes, A. doris, and A. ravifaux, as well as the four new species. The two species that are restricted to the highlands of Hispaniola (i.e., A. armouri and A. shrevei), are only considered in the respective diagnosis sections of the seven species treated here in detail. The same applies to those species that are only distantly related to those five species as evidenced by our genetic analyses (A. breslini, A. longitibialis, A. marcanoi, A. strahmi, and A. saxatilis).


Audantia cybotes (Cope, 1863)
Tiburon Stout Anole
Figs. 14–16
Anolis (Anolis) cybotes Cope, 1863: 177; type locality: Haiti, near Jeremie. Lectotype: MCZ 14346. Boulenger, 1885 (in part.); Schmidt, 1921 (in part.); Barbour & Loveridge, 1929
(in part.); Barbour, 1930a (in part.); Barbour, 1930b (in part.); Schwartz & Thomas, 1975 (in part.); Schwartz, 1979 (in part.); Wyles & Gorman, 1980 (in part.); Schwartz, 1980 (in part.); Henderson et al., 1984 (in part.); Henderson & Schwartz, 1984
(in part.); Burnell & Hedges, 1990 (in part.); Olson, 1990; Powell et al., 1996 (in part.); Queiroz et al., 1998 (in part.); Powell et al., 1999 (in part.); Poe, 2004 (in part.); Nicholson et al., 2005 (in part.); Henderson & Powell, 2009 (in part.); Boistel et al., 2011 (in part.); Kolbe et al., 2011
(in part.); Poe, 2013 (in part.); Köhler, 2014 (in part.); Muñoz et al., 2014a (in part.); Klaczko et al., 2015 (in part.); Giovannotti et al., 2017 (in part.); Poe et al., 2017 (in part.); Barbour, 1914 (in part.); Schwartz, 1989 (in part.).
Anolis cybotes cybotes: Cochran, 1934 (in part.); Barbour, 1935 (in part.); Barbour, 1937 (in part.); Mertens, 1938 (in part.); Mertens, 1939 (in part.); Cochran, 1941 (in part.); Schwartz & Thomas, 1975 (in part.); MacLean et al., 1977 (in part.); Henderson & Schwartz, 1984 (in part.); Henderson et al., 1984 (in part.); Schwartz & Henderson, 1991 (in part.); Fobes et al., 1993 (in part.); Powell et al., 1999 (in part.); Powell & Henderson, 2012 (in part.).
Anolis cybotes haetianus: Schwartz & Thomas, 1975; MacLean et al., 1977; Henderson & Schwartz, 1984, Henderson et al., 1984.
Anolis haetianus: Garman, 1887; Schwartz & Henderson, 1982; Barbour, 1914; Schwartz, 1989; Powell et al., 1996; Powell et al., 1999.
Anolis riisei Reinhardt & Lütken 1863: 264; type locality: “Haiti”. Lectotype: NHMD R3796. Audantia cybotes cybotes: Nicholson et al., 2012 (in part.); Nicholson et al., 2018 (in part.).
Audantia cybotes: Nicholson et al., 2014 (in part.).
Audantia haetiana: Nicholson et al., 2012; Nicholson et al., 2018. An incorrect spelling, because the original species name is a noun that does not change gender.
Ctenonotus cybotes: Savage & Guyer, 1989 (in part.).
Ctenonotus haetianus: Savage & Guyer, 1989.
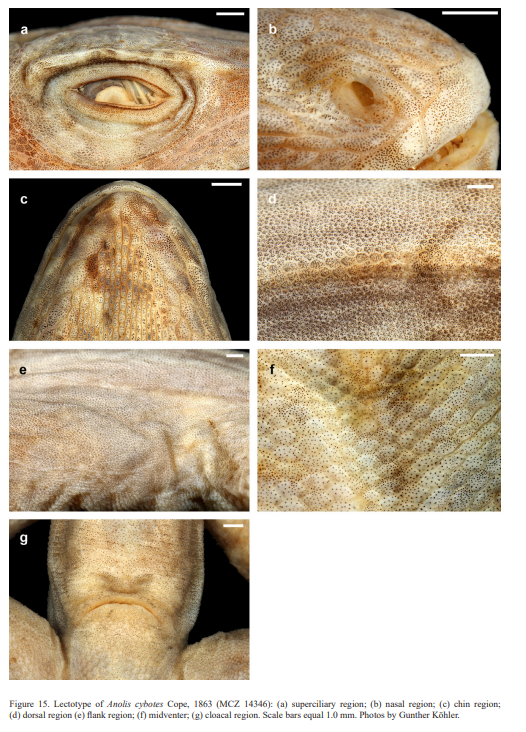
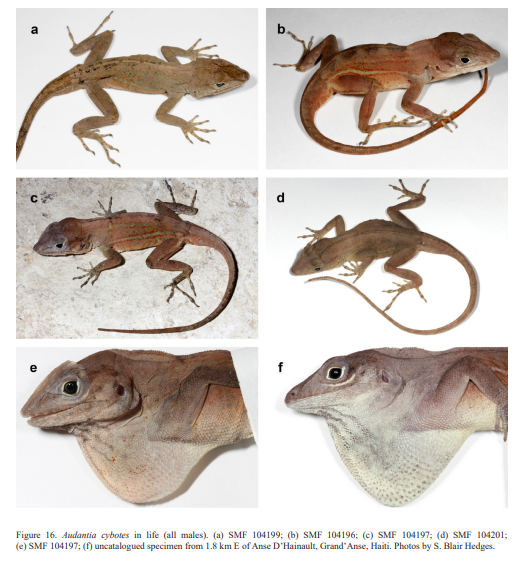
Diagnosis. A species of the genus Audantia that differs from all congeners by the combination of having (1) usually keeled ventral scales; (2) male dewlap dirty white without yellowish or orange suffusions, and with homogeneously distributed, narrowly spaced gorgetal scales, all large on posterior half of dewlap; (3) dark gular streaks in males present (Fig. 16e, f); (4) no patch of enlarged scales in nuchal region; (5) a double row of greatly enlarged (at least three times the size of adjacent scales), keeled and mucronate vertebral scales; (6) usually two sublabial scales in contact with infralabials; (7) 180–222 scales around midbody in males; and (8) keeled scales on dorsal surfaces of upper forelimb and anterior surface of thigh.
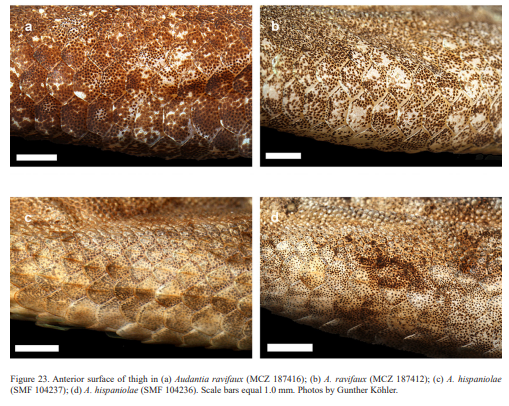
Audantia cybotes differs from A. armouri, A. breslini, A. shrevei, and A. saxatilis by having dark gular streaks (vs. usually absent); by having a double row of greatly enlarged, at least three times the size of adjacent scales, keeled and mucronate vertebral scales (vs. those scales only weakly enlarged, usually less than twice the size of adjacent scales, non-mucronate); and by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent). Audantia cybotes differs further from A. armouri by having 180–222 scales around midbody in males (vs. 118–172). Audantia cybotes differs further from A. shrevei by lacking a patch of greatly enlarged scales in nuchal region (vs. such a patch present). Audantia cybotes differs from A. doris by having dark gular streaks (vs. usually absent); by having a male dewlap with homogeneously distributed, narrowly spaced gorgetal scales, all large on posterior half of dewlap (vs. heterogeneously distributed with groups of cluttered scales, scales reduced in size in central portion of dewlap); and by having 180–222 scales around midbody in males (vs. 166–184). Audantia cybotes differs from A. marcanoi and A. strahmi by having a dirty white male dewlap (vs. rose-red at the edge, more orangish anteriorly and posteriorly, but purplish or even bluish toward the center in A. marcanoi, and orange with paler center in A. strahmi). Audantia cybotes differs further from A. marcanoi by having a double row of abruptly and greatly enlarged, at least three times the size of adjacent scales, keeled vertebral scales (vs. vertebral scales gradually and weakly enlarged, not forming a regular double row). Audantia cybotes differs from A. longitibialis by having usually keeled ventral scales (vs. smooth); and a male dewlap dirty white without yellowish or orange suffusions, and with homogeneously distributed, narrowly spaced gorgetal scales, all large on posterior half of dewlap (vs. heterogeneously distributed, somewhat spaced, and with groups of cluttered scales, scales smaller in central region of dewlap or all gorgetals small). Audantia cybotes differs from A. ravifaux by having keeled ventrals (vs. smooth); by having keeled scales on dorsal surface of upper forelimb and anterior surface of thigh (vs. smooth); by having a double row of greatly enlarged, at least three times the size of adjacent scales, keeled and mucronate vertebral scales (vs. those scales only weakly enlarged, usually less than twice the size of adjacent scales, smooth and non-mucronate); and by having homogeneously distributed, narrowly spaced gorgetal scales, all large on posterior half of dewlap (vs. heterogeneously distributed, somewhat spaced, and with groups of cluttered scales, scales smaller in central region of dewlap). For differences between A. cybotes and the species described below, see the respective accounts of the new species.
Description of lectotype. Adult male, as indicated by well-developed dewlap, and presence of a pair of greatly enlarged postcloacal scales (Figs. 14–15); SVL 64.0 mm; tail incomplete; axilla to groin distance 20.4 mm; head length 20.0 mm, head length/SVL ratio 0.31; snout length 8.6 mm; head width 10.8 mm; shank length 18.3 mm, shank length/head length ratio 0.92. Dorsal head scales smooth or rugose, except weakly keeled scales on snout and supraoculars; 7 postrostrals; 6 scales between nasals; 1 elongate prenasal scale on each side, distinct from circumnasal and in contact with both rostral and first supralabial; circumnasal separated from first supralabial by one scale; scales in deep prefrontal depression smooth or rugose; supraorbital semicircles welldeveloped, broadly in contact medially; supraorbital disc composed of 3 moderately enlarged, keeled scales arranged in two rows; circumorbital row incomplete, therefore, some enlarged supraorbital scales contacting supraorbital semicircles; a very large elongated superciliary, followed posteriorly by a much smaller, overlapping one and by several small, keeled scales; 2–3 rows of small keeled scales extending between enlarged supraorbitals and large superciliary; a deep parietal depression present; interparietal scale well-developed, 2.5 x 1.5 mm (length x width), surrounded by scales of moderate size; 2 scales present between interparietal and supraorbital semicircles; canthal ridge distinct, composed of 3 large and 2 small anterior canthal scales; 7 scales present between second canthals; 9 scales present between posterior canthals; 74 (right) mostly keeled loreal scales in a maximum of 9 (right) horizontal rows; 8 keeled subocular scales arranged in a single row; 6 supralabials to level below center of eye; suboculars separated from supralabials by one scale row; ear opening 1.4 x 1.9 mm (length x height); mental distinctly wider than long, almost completely divided medially, bordered posteriorly by 7 postmentals, outer ones much larger than median ones; 6 infralabials to level below center of eye; sublabials greatly enlarged (< four times the size of medial postmental scales), 2 in contact with infralabials; scales in sublabial row much larger than scales medially adjacent to this row; pointed granular scales present on chin and throat; dewlap large, extending from level below anterior margin of eye onto chest; a nuchal crest and a dorsal ridge present; dorsum of body with mostly smooth, some weakly keeled, granular scales; 2 medial rows slightly enlarged, usually at least twice the size of adjacent scales; about 54 medial dorsal scales in one head length; about 62 medial dorsal scales between levels of axilla and groin; lateral scales mostly smooth, granular and more or less homogeneous in size; ventrals at midbody smooth, flat, almost cycloid, subimbricate; about 36 medial ventral scales in one head length; about 40 medial ventral scales between levels of axilla and groin; 186 scales around midbody; ventral basal caudal scales smooth; a pair of greatly enlarged postcloacal scales present, about 1.5 mm wide; no tube-like axillary pocket present; scales on dorsal surface of upper forelimb keeled, imbricate; scales on anterior surface of thigh enlarged, keeled, imbricate; digital pads dilated, dilated pad three times the width of non-dilated distal phalanx; distal phalanx narrower than and raised from dilated pad; 34 lamellae under Phalanges II–IV of Toe IV of hind limbs; 9 scales under distal phalanx of Toe IV of hind limbs.
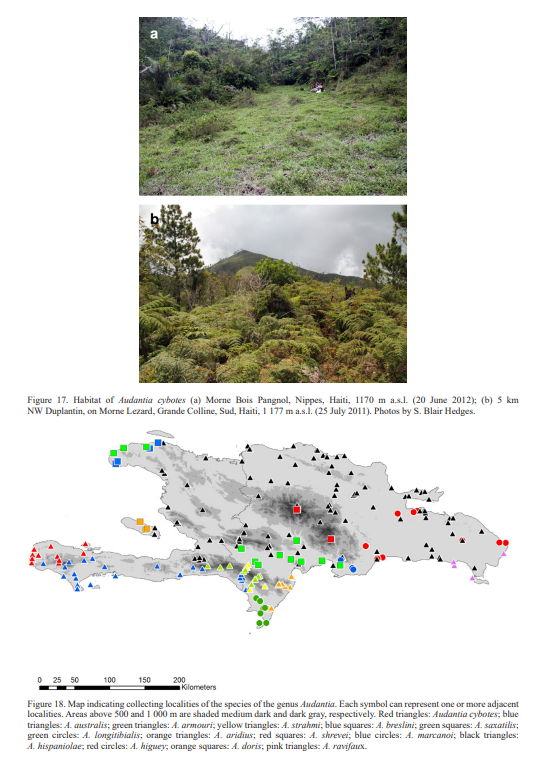
Geographic distribution. As currently known, Audantia cybotes is restricted to the western portion of the Tiburón Peninsula, Haiti, from near sea level to 1–780 m a. s. l. (Fig. 18).
Natural history notes. Audantia cybotes seems to be quite adaptable in regard of the tolerated habitats and even seems to prefer disturbed habitat, forest edges, and villages as long as trees and bushes provide shade and humidity (Fig. 17). At night these lizards sleep on leafs and twigs 1 to 2 m above the ground but have also been found under rocks and logs. Henderson & Powell (2009) provided a summary of the natural history of “Anolis cybotes” which under our concept is represented by several species.
Conservation. Given its usual abundance wherever this species occurs, we consider the conservation status of Audantia cybotes as Least Concern based on the IUCN Red List Categories and Criteria (IUCN, 2012).
Audantia doris (Barbour, 1925) Gonave Stout Anole Figs. 19–21 Anolis cybotes: Boulenger, 1885 (in part.); Schmidt, 1921 (in part.); Schwartz, 1979 (in part.); Wyles & Gorman, 1980 (in part.); Schwartz, 1980 (in part.); Henderson et al., 1984 (in part.); Schwartz, 1989 (in part.); Burnell & Hedges, 1990 (in part.); Powell et al., 1996 (in part.); Queiroz et al., 1998 (in part.); Poe, 2004 (in part.); Nicholson et al., 2005 (in part.); Henderson & Powell, 2009 (in part.); Boistel et al., 2011 (in part.); Kolbe et al., 2011 (in part.); Poe, 2013 (in part.); Köhler, 2014 (in part.); Muñoz et al., 2014 a (in part.); Klaczko et al., 2015 (in part.); Giovannotti et al., 2017 (in part.); Poe et al., 2017 (in part.). Anolis doris Barbour, 1925: 101; type locality: Île de la Gonâve. Holotype: MCZ 13739. Cochran, 1928, Barbour & Loveridge, 1929; Barbour, 1930a; Barbour, 1930b. Anolis cybotes doris: Cochran, 1934; Barbour, 1935; Barbour, 1937; Cochran, 1941; Schwartz & Thomas, 1975 (in part.); MacLean et al., 1977; Schwartz et al., 1982; Henderson & Schwartz, 1984; Henderson et al., 1984 (in part.); Schwartz & Henderson, 1991; Fobes et al., 1993; Powell et al., 1999; Powell & Henderson, 2012. Audantia cybotes: Nicholson et al., 2014 (in part.). Audantia cybotes doris: Nicholson et al., 2012; Nicholson et al., 2018. Ctenonotus cybotes: Savage & Guyer, 1989 (in part.). Diagnosis. A species of the genus Audantia that differs from all congeners by the combination of having (1) smooth ventral scales; (2) male dewlap creme white with central orange blotch (Fig. 21b), and with heterogeneously distributed, intermediate spaced gorgetal scales, that are cluttered in groups, and reduced in size in central portion; (3) no dark gular streaks in males; (4) no patch of enlarged scales in nuchal region; (5) a double row of greatly enlarged (at least three times the size of adjacent scales), keeled and mucronate vertebral scales; (6) usually two sublabial scales in contact with infralabials; (7) 166–184 scales around midbody in males; and (8) keeled scales on dorsal surfaces of upper forelimb and anterior surface of thigh. Audantia doris differs from A. breslini, A. cybotes, A. shrevei, and A. saxatilis by having smooth ventrals (vs. keeled, some individuals of A. breslini and A. cybotes with smooth ventrals); by having an orange blotch in the center on the male dewlap (vs. absent); and by having heterogeneously distributed gorgetals with groups of cluttered scales (vs. homogeneously distributed gorgetals). Audantia doris differs further from A. shrevei by lacking a patch of greatly enlarged scales in nuchal region (vs. such a patch present). Audantia doris differs from A. armouri by having a double row of greatly enlarged, at least three times the size of adjacent scales, keeled and mucronate vertebral scales (vs. those scales only weakly enlarged, usually less than twice the size of adjacent scales, non-mucronate); by having 166–184 scales around midbody in males (vs. 118–172); by having an orange blotch arranged in the center on the male dewlap (vs. absent); and by having heterogeneously distributed gorgetals with groups of cluttered scales (vs. homogeneously distributed gorgetals). Audantia doris differs from A. cybotes by having no dark gular streaks (present); by having the gorgetal scales reduced in size in central portion of posterior half of male dewlap (vs. all gorgetals large on posterior half of dewlap); and by having heterogeneously distributed gorgetals with groups of cluttered scales (vs. homogeneously distributed gorgetals). Audantia doris differs from A. marcanoi and A. strahmi by having a creme white male dewlap with an orange blotch arranged in the center (vs. rose-red at the edge, more orangish anteriorly and posteriorly, but purplish or even bluish toward the center in A. marcanoi, and orange with paler center in A. strahmi). Audantia doris differs from A. longitibialis by having a creme white dewlap with a central orange blotch (vs. yellow without central orange blotch); and by having mucronate middorsals (vs. non-mucronate middorsals). Audantia doris differs from A. ravifaux by having keeled scales on dorsal surface of upper forelimb and anterior surface of thigh (vs. smooth); by having a double row of greatly enlarged, at least three times the size of adjacent scales, keeled and mucronate vertebral scales (vs. those scales only weakly enlarged, usually less than twice the size of adjacent scales, smooth and non-mucronate); and by having a male dewlap with an orange blotch arranged in the center (vs. absent). For differences between A. doris and the species described below, see the respective accounts of the new species. Description of lectotype. Adult male, as indicated by well-developed dewlap, and presence of a pair of greatly enlarged postcloacal scales (Figs. 19–20); SVL 59.0 mm; tail length 105 mm (complete); axilla to groin distance 18.1 mm; head length 18.9 mm, head length/SVL ratio 0.32; snout length 8.3 mm; head width 10.3 mm; shank length 18.7 mm, shank length/ head length ratio 0.99. Dorsal head scales smooth or rugose, except weakly keeled scales on snout and supraoculars; 5 postrostrals; 6 scales between nasals; 1 elongate prenasal scale on each side, distinct from circumnasal and in contact with both rostral and first supralabial; circumnasal in contact with first supralabial; scales in moderate prefrontal depression smooth or rugose; supraorbital semicircles well-developed, broadly in contact medially; supraorbital disc composed of 3 moderately enlarged, keeled scales arranged in two rows; circumorbital row complete, therefore, enlarged supraorbital scales separated from supraorbital semicircles; a very large elongated superciliary, followed posteriorly by a much smaller, overlapping one and by several small, keeled scales; 2–3 rows of small keeled scales extending between enlarged supraorbitals and large superciliary; a shallow parietal depression present; interparietal scale well-developed, 2.4 x 0.7 mm (length x width), surrounded by scales of moderate size; 2 scales present between interparietal and supraorbital semicircles; canthal ridge distinct, composed of 3 large and 2 small anterior canthal scales; 6 scales present between second canthals; 9 scales present between posterior canthals; 38 (both sides) mostly keeled loreal scales in a maximum of 7 (both sides) horizontal rows; 7 keeled subocular scales arranged in a single row; 7 supralabials to level below center of eye; suboculars separated from supralabials by one scale row; ear opening 1.5 x 1.9 mm (length x height); mental distinctly wider than long, almost completely divided medially, bordered posteriorly by 6 postmentals, outer ones much larger than median ones; 6 infralabials to level below center of eye; sublabials greatly enlarged (< four times the size of medial postmental scales), 2 in contact with infralabials; scales in sublabial row much larger than scales medially adjacent to this row; pointed granular scales present on chin and throat; dewlap large, extending from level below anterior margin of eye onto chest; a nuchal crest and a dorsal ridge present; dorsum of body with mostly smooth, some weakly keeled, granular scales; 2 medial rows slightly enlarged, usually at least twice the size of adjacent scales; about 34 medial dorsal scales in one head length; about 46 medial dorsal scales between levels of axilla and groin; lateral scales mostly smooth, granular and more or less homogeneous in size; ventrals at midbody smooth, flat, almost cycloid, subimbricate; about 40 medial ventral scales in one head length; about 41 medial ventral scales between levels of axilla and groin; 180 scales around midbody; ventral basal caudal scales smooth, all other caudal scales keeled; middorsal caudal scales distinctly enlarged, forming a low crest; lateral caudal scales with whorls of enlarged scales; a pair of greatly enlarged postcloacal scales present, about 1.7 mm wide; no tube-like axillary pocket present; scales on dorsal surface of upper forelimb keeled, imbricate; scales on anterior surface of thigh enlarged, keeled, imbricate; digital pads dilated, dilated pad three times the width of non-dilated distal phalanx; distal phalanx narrower than and raised from dilated pad; 26 lamellae under Phalanges II–IV of Toe IV of hind limbs; 8 scales under distal phalanx of Toe IV of hind limbs. Geographic distribution. As currently known, Audantia doris is restricted to Île de la Gonâve, Haiti, from near sea level to 445 m a. s. l. (Fig. 18). Natural history notes. Henderson & Powell (2009) provided a summary of the natural history of “Anolis cybotes” which under our concept is represented by several species. SBH collected this species in various habitats on Île de la Gonâve, Haiti, including open coastal vegetation with coconut palm trees (Fig. 22). Conservation. Given that two closely related species of the genus Audantia occur on Île de la Gonâve (i.e., A. doris and a species described below) it is possible that gene introgression between these two species occurs as indicated by the intermediate morphology of the Gonave specimens of the species described below compared to their mainland conspecifics. Audantia ravifaux (Schwartz & Henderson, 1982) Saona Stout Anole Figs. 23–26 Anolis cybotes: Boulenger, 1885 (in part.); Schmidt, 1921 (in part.); Barbour & Loveridge, 1929 (in part.); Barbour, 1930a (in part.); Barbour, 1930b (in part.); Schwartz, 1979 (in part.); Wyles & Gorman, 1980 (in part.); Schwartz, 1980 (in part.); Henderson et al., 1984 (in part.); Schwartz, 1989 (in part.); Burnell & Hedges, 1990 (in part.); Powell et al., 1996 (in part.); Queiroz et al., 1998 (in part.); Poe, 2004 (in part.); Nicholson et al., 2005 (in part.); Henderson & Powell, 2009 (in part.); Boistel et al., 2011 (in part.); Kolbe et al., 2011 (in part.); Poe, 2013 (in part.); Köhler, 2014 (in part.); Muñoz et al., 2014a (in part.); Klaczko et al., 2015 (in part.); Giovannotti et al., 2017 (in part.); Poe et al., 2017 (in part.). Anolis cybotes ravifaux Schwartz & Henderson, 1982: 3; type locality: environs of Mano Juan, Isla Saona, República Dominicana. Holotype: MCZ 156221. Schwartz et al., 1982; Henderson & Schwartz, 1984; Henderson et al., 1984 (in part.); Schwartz & Henderson, 1991; Powell et al., 1999; Powell & Henderson, 2012. Anolis cybotes cybotes: Cochran, 1934 (in part.); Barbour, 1935 (in part.); Barbour, 1937 (in part.); Fobes et al., 1993 (in part.); Cochran, 1941 (in part.); MacLean et al., 1977 (in part.). Audantia cybotes ravifaux: Nicholson et al., 2012; Nicholson et al., 2018. Audantia cybotes: Nicholson et al., 2014 (in part.). Ctenonotus cybotes: Savage & Guyer, 1989 (in part.). Diagnosis. A species of the genus Audantia that differs from all congeners by the combination of having (1) smooth ventral scales; (2) male dewlap dirty white with or without yellow suffusions; (3) no dark gular streaks in males; (4) no patch of enlarged scales in nuchal region; (5) a double row of weakly enlarged (usually less than twice the size of adjacent scales), smooth and non-mucronate vertebral scales; (6) two or three sublabial scales in contact with infralabials; (7) 170–204 scales around midbody in males; and (8) enlarged scales on anterior surface of thigh smooth (Fig. 23). Audantia ravifaux differs from A. breslini, A. cybotes, A. saxatilis, and A. shrevei by having smooth enlarged scales on anterior surface of thigh (vs. keeled); and by having smooth ventrals (vs. keeled, some individuals of A. breslini and A. cybotes with smooth ventrals). Audantia ravifaux differs further from A. shrevei by lacking a patch of greatly enlarged scales in nuchal region (vs. such a patch present). Audantia ravifaux differs from A. marcanoi and A. strahmi by having smooth enlarged scales on anterior surface of thigh (vs. keeled); and by having a dirty white male dewlap (vs. rose-red at the edge, more orangish anteriorly and posteriorly, but purplish or even bluish toward the center in A. marcanoi, and orange with paler center in A. strahmi). Audantia ravifaux differs from A. armouri, A. doris, and A. longitibialis by having smooth enlarged scales on anterior surface of thigh (vs. keeled). Audantia ravifaux differs further from A. doris by having a double row of weakly enlarged, usually less than twice the size of adjacent scales, smooth and non-mucronate vertebral scales (vs. greatly enlarged, at least three times the size of adjacent scales, keeled and mucronate vertebral scales). For differences between A. ravifaux and the species described below, see the respective accounts of the new species. Description of holotype. Adult male, as indicated by well-developed dewlap, and presence of a pair of greatly enlarged postcloacal scales (Figs. 24 and 25); SVL 58.0 mm; tail length 83.0 mm (incomplete); tail distinctly compressed in cross section, tail height 3.2 mm and width 1.8 mm; axilla to groin distance 21.7 mm; head length 18.9 mm, head length/SVL ratio 0.33; snout length 8.4 mm; head width 10.5 mm; longest toe of adpressed hind limb reaching to point between eye and nostril; shank length 18.2 mm, shank length/head length ratio 0.96; longest finger of extended forelimb reaching to 3 mm beyond tip of snout; longest finger of adpressed forelimb reaching to 4 mm past anterior insertion of hind limbs. Dorsal head scales smooth or rugose, except weakly keeled scales on snout and supraoculars; 5 postrostrals; 6 scales between nasals; 1 elongate prenasal scale on each side, distinct from circumnasal and in contact with both rostral and first supralabial; circumnasal in contact with first supralabial; scales in deep prefrontal depression smooth or rugose; supraorbital semicircles well-developed, broadly in contact medially; supraorbital disc composed of 5 moderately enlarged, keeled scales arranged in two rows; circumorbital row complete, therefore, enlarged supraorbital scales separated from supraorbital semicircles; a very large elongated superciliary, followed posteriorly by a much smaller, overlapping one, and by three small, keeled scales; 2-3 rows of small keeled scales extending between enlarged supraorbitals and large superciliary; a deep parietal depression present; interparietal scale well-developed, 2.3 x 1.3 mm (length x width), surrounded by scales of moderate size; 2 scales present between interparietal and supraorbital semicircles; canthal ridge distinct, composed of 3 large and 2 small anterior canthal scales; 5 scales present between second canthals; 8 scales present between posterior canthals; 35 (right) mostly keeled loreal scales in a maximum of 7 (right) horizontal rows; 8 keeled subocular scales arranged in a single row; 7 supralabials to level below center of eye; suboculars narrowly in contact with supralabials; ear opening 1.5 x 2.2 mm (length x height); mental distinctly wider than long, almost completely divided medially, bordered posteriorly by 8 postmentals, outer ones much larger than median ones; 6 infralabials to level below center of eye; sublabials greatly enlarged (< four times the size of medial postmental scales), 3 (right)–4 (left) in contact with infralabials; scales in sublabial row much larger than scales medially adjacent to this row; pointed granular scales present on chin and throat; dewlap large, extending from level below anterior margin of eye onto chest; a nuchal crest and a dorsal ridge present; dorsum of body with mostly smooth, some weakly keeled, granular scales; 2 medial rows slightly enlarged, usually less than twice the size of adjacent scales; largest dorsal scales about 0.50 x 0.30 mm (length x width); about 45 medial dorsal scales in one head length; about 61 medial dorsal scales between levels of axilla and groin; lateral scales keeled, granular and more or less homogeneous in size, average size 0.15 mm in diameter; ventrals at midbody smooth, flat, almost cycloid, subimbricate, about 0.40 x 0.60 mm (length x width); about 42 medial ventral scales in one head length; about 49 medial ventral scales between levels of axilla and groin; 170 scales around midbody; ventral basal caudal scales smooth, all other caudal scales keeled; middorsal caudal scales distinctly enlarged, forming a low crest; lateral caudal scales with whorls of enlarged scales; a pair of greatly enlarged postcloacal scales present, about 1.1 mm wide; no tube-like axillary pocket present; scales on dorsal surface of upper forelimb smooth, imbricate; scales on anterior surface of thigh enlarged, smooth, imbricate; digital pads dilated, dilated pad three times the width of non-dilated distal phalanx; distal phalanx narrower than and raised from dilated pad; 30 lamellae under Phalanges II–IV of Toe IV of hind limbs; 9 scales under distal phalanx of Toe IV of hind limbs. Coloration after 45 years preservation in 70 % ethanol was recorded as follows: Dorsal surfaces of head, body, limbs, and tail Walnut Brown (27); ventral surface of head Clay Color (18) except for Beige (254) dewlap; ventral surface of body Clay Color (18) with a suffusion of Drab (19); ventral surfaces of legs Clay Color (18) with Prout’s Brown (47) speckles; ventral surface of tail Clay Color (18). The completely everted hemipenis (SMF 97873; Fig. 26) is a medium-sized, slightly bilobate organ; sulcus spermaticus bordered by well-developed sulcal lips and opening into a single large apical field void of ornamentation; a low asulcate ridge present; apex strongly calyculate, truncus with transverse folds. The everted hemipenis of another specimen (SMF 97869) agrees well with this description. Geographic distribution. As currently known, Audantia ravifaux is restricted to the extreme southeastern portion of Hispaniola including Isla Saona, all from near sea level (Fig. 18). Natural history notes. Henderson & Powell (2009) provided a summary of the natural history of “Anolis cybotes” which under our concept is represented by several species. Conservation. More field and lab work is needed to evaluate the geographic area of distribution of Audantia ravifaux in order to understand the status of conservation of this species. Audantia hispaniolae sp. nov. Common Stout Anole ZOOBANK urn: lsid: zoobank.org: act:5FFDB3F3-D7AC-4638-B25F-D493BE1CE80B. Figs. 29–34 Anolis cybotes: Boulenger, 1885 (in part.); Barbour, 1914 (in part.); Schmidt, 1921 (in part.); Noble, 1923b; Cochran, 1928 (in part.); Barbour & Loveridge, 1929 (in part.); Barbour, 1930a (in part.); Barbour, 1930b (in part.); Rand, 1962 (in part.); Williams, 1963 (in part.); Schwartz & Thomas, 1975 (in part.); Williams, 1975 (in part.); Schwartz, 1979 (in part.); Wyles & Gorman, 1980 (in part.); Schwartz, 1980 (in part.); Schwartz & Henderson, 1982 (in part.); Henderson et al., 1984 (in part.); Henderson & Schwartz, 1984 (in part.); (Losos, 1985) (in part.); Case & Williams, 1987; Schwartz, 1989 (in part.); Burnell & Hedges, 1990 (in part.); Powell et al., 1996 (in part.); Queiroz et al., 1998 (in part.); Powell et al., 1999 (in part.); Poe, 2004 (in part.); Nicholson et al., 2005 (in part.); Henderson & Powell, 2009 (in part.); Boistel et al., 2011 (in part.); Kolbe et al., 2011 (in part.); Poe, 2013 (in part.); Wollenberg et al., 2013 (in part.); Köhler, 2014 (in part.); Muñoz et al., 2014a (in part.); Muñoz et al., 2014b (in part.); Klaczko et al., 2015 (in part.); Giovannotti et al., 2017 (in part.); Poe et al., 2017 (in part.); Boronow et al., 2018 (in part.); Muñoz & Losos, 2018 (in part.). Anolis cybotes cybotes: Cochran, 1934 (in part.); Barbour, 1935 (in part.); Barbour, 1937 (in part.); Mertens, 1938 (in part.); Mertens, 1939 (in part.); Cochran, 1941 (in part.); Schwartz & Thomas, 1975 (in part.); MacLean et al., 1977 (in part.); Schwartz & Henderson, 1991 (in part.); Fobes et al., 1993 (in part.); Powell et al., 1999 (in part.); Powell & Henderson, 2012 (in part.). Audantia cybotes: Nicholson et al., 2014 (in part.); Nicholson et al., 2012 (in part.); Nicholson et al., 2018 (in part.). Ctenonotus cybotes: Savage & Guyer, 1989 (in part.). Holotype. SMF 97884, an adult male from El Limón, Peninsula Samaná (19.28929, -69.43118), 30 m, Province Samaná, Dominican Republic; collected 21 October 2013 by Gunther Köhler. Field tag number GK-4721. Paratypes. MNHNSD 23.3602–03, SMF 97878–83, 97885, same collecting data as holotype. All paratypes are adult males except MNHNSD 23.3602, SMF 97878, 97881, 97885 that are adult females. Diagnosis. A species of the genus Audantia (our Species 1 “hispaniolae”) that differs from all congeners by the combination of having (1) smooth ventral scales; (2) male dewlap creme white with yellowish, greenish or orange suffusions, and with heterogeneously distributed, moderately spaced gorgetal scales, that are clutterd in groups, and reduced in size in central portion; (3) dark gular streaks in males present; (4) no patch of enlarged scales in nuchal region; (5) a double row of enlarged, keeled and non-mucronate vertebral scales; (6) usually two sublabial scales in contact with infralabials; (7) 214–244 scales around midbody in males; and (8) keeled scales on dorsal surfaces of upper forelimb and anterior surface of thigh. Audantia hispaniolae differs from A. breslini, A. shrevei, and A. saxatilis by having smooth ventrals (vs. keeled, some individuals of A. breslini with smooth ventrals); by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent); by having heterogeneously distributed gorgetals with groups of cluttered scales (vs. homogeneously distributed gorgetals); and by having dark gular streaks on male dewlap (vs. usually absent). Audantia hispaniolae differs further from A. shrevei by lacking a patch of greatly enlarged scales in nuchal region (vs. such a patch present). Audantia hispaniolae differs from A. armouri by having dark gular streaks on the male dewlap (vs. usually absent); by having heterogeneously distributed gorgetals with groups of cluttered scales (vs. homogeneously distributed gorgetals); and by having 214–240 scales around midbody in males (vs. 118–172). Audantia hispaniolae differs from A. cybotes by having smooth ventrals (vs. keeled, smooth in some individuals); by having non-mucronate vertebral scales (vs. vertebral scales mucronate); by having heterogeneously distributed gorgetals with groups of cluttered scales (vs. homogeneously distributed gorgetals); by having moderately spaced gorgetals and scales reduced in size in central portion of dewlap (vs. all scales narrowly spaced and large on posterior half of dewlap); and males having a dirty white dewlap with yellowish, greenish or orange suffusions (vs. those suffusions absent). Audantia hispaniolae differs from A. doris by having dark gular streaks (vs. absent); by having yellowish, greenish or orange suffusions on male dewlap (vs. no suffusions, but with an orange blotch in center of dewlap); and by having 214–244 scales around midbody in males (vs. 166184). Audantia hispaniolae differs from A. marcanoi and A. strahmi by having a dirty white male dewlap (vs. rose-red at the edge, more orangish anteriorly and posteriorly, but purplish or even bluish toward the center in A. marcanoi, and orange with paler center in A. strahmi). Audantia hispaniolae differs further from A. marcanoi by having a double row of abruptly enlarged vertebral scales (vs. vertebral scales gradually enlarged, not forming a regular double row). Audantia hispaniolae differs from A. longitibialis by having dark gular streaks on male dewlap (vs. absent); and by having moderately spaced gorgetals and scales reduced in size in central portion of dewlap (vs. all scales widely spaced and small on posterior half of dewlap). Audantia hispaniolae differs from A. ravifaux by having keeled scales on dorsal surface of upper forelimb and anterior surface of thigh (vs. smooth); by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent); and by having moderately spaced gorgetals (vs. all scales widely spaced). For differences between A. hispaniolae and the species described below, see the respective accounts of the new species. Description of the holotype. Adult male (Figs. 29 and 30), as indicated by everted hemipenes, well-developed dewlap, and presence of a pair of greatly enlarged postcloacal scales; SVL 62.0 mm; tail length 52.0 mm (incomplete); tail distinctly compressed in cross section, tail height 3.9 mm and width 2.5 mm; axilla to groin distance 22.7 mm; head length 18.6 mm, head length/SVL ratio 0.30; snout length 8.2 mm; head width 10.7 mm; longest toe of adpressed hind limb reaching to anterior margin of eye; shank length 19.1 mm, shank length/head length ratio 1.03; longest finger of extended forelimb reaching to nostril; longest finger of adpressed forelimb reaching to level of anterior insertion of hind limbs. Dorsal head scales smooth or rugose, except weakly keeled scales on snout and supraoculars; 6 postrostrals; 7 scales between nasals; 1 elongate prenasal scale on each side, distinct from circumnasal and in contact with both rostral and first supralabial; circumnasal separated from first supralabial by one scale; scales in deep prefrontal depression smooth or rugose; supraorbital semicircles well-developed, separated medially by one scale row at narrowest point; supraorbital disc composed of 3 moderately enlarged, keeled scales arranged in three rows; circumorbital row complete, therefore, enlarged supraorbital scales separated from supraorbital semicircles; a very large elongated superciliary, followed posteriorly by a much smaller, overlapping one and by several small, keeled scales; three rows of small keeled scales extending between enlarged supraorbitals and large superciliary; a deep parietal depression present; interparietal scale well-developed, 2.5 x 1.4 mm (length x width), surrounded by scales of moderate size; 2 to 3 scales present between interparietal and supraorbital semicircles; canthal ridge distinct, composed of 3 large and 2 small anterior canthal scales; 9 scales present between second canthals; 11 scales present between posterior canthals; 73 (right) – 62 (left) mostly keeled loreal scales in a maximum of 8 horizontal rows; 7 to 8 keeled subocular scales arranged in a single row; 7 supralabials to level below center of eye; suboculars separated from supralabials by a complete scale row; ear opening 1.3 x 2.5 mm (length x height); mental distinctly wider than long, almost completely divided medially, bordered posteriorly by 5 postmentals, outer ones much larger than median ones; 7 infralabials to level below center of eye; sublabials greatly enlarged (< four times the size of medial postmental scales), two in contact with infralabials; scales in sublabial row much larger than scales medially adjacent to this row; pointed granular scales present on chin and throat; dewlap large, extending from level below anterior margin of eye onto chest; gorgetals heterogeneously distributed with groups of cluttered scales, those in central portion reduced in size; a nuchal crest and a dorsal ridge present; dorsum of body with keeled, granular scales; 2 medial rows distinctly enlarged, mostly more than twice the size of adjacent body scales; largest dorsal scales about 0.35 x 0.30 mm (length x width); about 56 medial dorsal scales in one head length; about 77 medial dorsal scales between levels of axilla and groin; lateral scales keeled, granular and more or less homogeneous in size, average size 0.12 mm in diameter; ventrals at midbody smooth, flat, almost cycloid, imbricate, about 0.65 x 0.75 mm (length x width); about 36 medial ventral scales in one head length; about 42 medial ventral scales between levels of axilla and groin; 236 scales around midbody; all caudal scales keeled; middorsal caudal scales distinctly enlarged, forming a low crest; lateral caudal scales with whorls of enlarged scales; a pair of greatly enlarged postcloacal scales present, about 1.2 mm wide; no tube-like axillary pocket present; scales on dorsal surface of upper forelimb strongly keeled, mucronate, imbricate; scales on anterior surface of thigh strongly keeled, mucronate, imbricate; digital pads dilated, dilated pad three times the width of non-dilated distal phalanx; distal phalanx narrower than and raised from dilated pad; 28 lamellae under Phalanges II-IV of Toe IV of hind limbs; 11 scales under distal phalanx of Toe IV of hind limbs. Coloration after 5 years preservation in 70% ethanol was recorded as follows: Dorsal ground color Smoke Gray (267) with Vandyke Brown (281) diffuse blotches; ventral surface of head Light Buff (2) with Hair Brown (277) streaks; ventral surface of body Cream White (52); ventral surfaces of legs Pale Pinkish Buff (3) with Hair Brown (277) stipples; ventral surface of fingers and toes Hair Brown (277); dorsal, lateral and ventral surfaces of tail Hair Brown (277). Variation. The paratypes agree well with the holotype in general appearance; morphometrics and scalation (Table II). In most individuals the longest toe of adpressed hind leg reaching to a point between anterior and posterior level of eye, exceptionally to a point between eye and nostril or between ear and eye. The coloration in life of an adult male (SMF 97863) was recorded as follows: Dorsum of head Ground Cinnamon (270); dorsum of body and limbs Ground Cinnamon (270), with a Light Smoke Grey (263) stripe edged by True Cinnamon (260); dewlap Whitish Lime Green (111), centrally suffused with Cinnamon (255); underside of head Beige (254) with Dark Drab (45) irregular lines; circum orbitale region Dark Greyish Olive (275) with a Pale Horn Color (11) inner ring; iris Maroon (39); ventral side of body and tail Pale Buff (1); ventral surface of limbs Cinnamon-Drab (50) with Prout’s Brown (47) speckles. The coloration in life of an adult female (SMF 97866) was recorded as follows: Dorsum of head Drab (19); dorsal surface of body Drab (19) with Tawny Olive (17) vertebral stripes and diamonds; lateral side of body with a Sayal Brown (41) longitudinal stripe; dorsal surface of limbs Verona Brown (37) with indistinct Drab (19) bands; tail Drab (19) with Clay Color (18) diamonds; iris Raw Umber (22); a Pale Horn Color (1) inner eye ring; ventral surface of head Smokey White (261) with Sepia (279) streaks; ventral suface of body and limbs Light Buff (2); ventral surface of tail Pale Pinkish Buff (3), grading into Cinnamon-Drab (50) distally. The completely everted hemipenis (SMF 104760; Fig. 31) is a medium-sized, slightly bilobate organ; sulcus spermaticus bordered by well-developed sulcal lips and opening into a single large apical field void of ornamentation; an asulcate finger-like processus present; apex strongly calyculate, truncus with transverse folds. The everted hemipenis of 19 other specimens (SMF 90421, 97863–65, 97868, 97875–76, 97882–84, 97888, 97900, 97903, 104758–62, 104764) agree well with this description. However, the shape of the asulcal processus varies from ridge-like to finger-like. Etymology. The species name (hispaniolae) is a feminine genitive singular noun, referring to the broad distribution of the species on the island of Hispaniola. Geographic distribution. As currently known, Audantia hispaniolae is widely distributed across the North Island of Hispaniola north of the Sierra de Bahoruco, except for the extreme northwestern portion, from near sea level to 1800 m a.s.l. (Fig. 18). Natural history notes. We observed this species in a wide range of habitats, ranging from wet cloud forest to degraded, rocky, dry forest, and also very often within human settlements as long as trees were present (Fig. 34). Individuals were collected on rocks, under rocks, low on tree trucks and bush stems, and under among dead agaves during the day. Especially in the morning hours, individuals were seen perching head-down on bushes and trees. At night we saw these lizards sleeping on leaves and twigs of palms, bushes and trees up to 2 m above the ground. In some instances, the same individual was observed on exactly the same sleeping place on several successive nights. The flight distance was usually in the range of 1–2 m. Both males and females often emitted a high-pitched distress call when caught. Henderson & Powell (2009) provided a summary of the natural history of “Anolis cybotes” which under our concept is represented by several species. Conservation. Given its broad range and usual abundance wherever this species occurs, we consider the conservation status of Audantia hispaniolae as Least Concern based on the IUCN Red List Categories and Criteria (IUCN, 2012). Audantia higuey sp. nov. Cordillera Oriental Stout Anole ZOOBANK urn:lsid:zoobank.org: act:F025F86B-79C4-41C2-A828-78C89317DFCC. Figs. 35–39 Anolis cybotes: Boulenger, 1885 (in part.); Schmidt, 1921 (in part.); Barbour & Loveridge, 1929 (in part.); Barbour, 1930a (in part.); Barbour, 1930b (in part.); Rand, 1962 (in part.); Schwartz & Thomas, 1975 (in part.); Schwartz, 1979 (in part.); Wyles & Gorman, 1980 (in part.); Schwartz, 1980 (in part.); Schwartz & Henderson, 1982 (in part.); Henderson et al., 1984 (in part.); Henderson & Schwartz, 1984 (in part.); Schwartz, 1989 (in part.); Burnell & Hedges, 1990 (in part.); Powell et al., 1996 (in part.); Queiroz et al., 1998 (in part.); Powell et al., 1999 (in part.); Poe, 2004 (in part.); Nicholson et al., 2005 (in part.); Henderson & Powell, 2009 (in part.); Boistel et al., 2011 (in part.); Kolbe et al., 2011 (in part.); Poe, 2013 (in part.); Köhler, 2014 (in part.); Muñoz et al., 2014 (in part.); Klaczko et al., 2015 (in part.); Giovannotti et al., 2017 (in part.); Poe et al., 2017 (in part.). Anolis cybotes cybotes: Cochran, 1934 (in part.); Barbour, 1935 (in part.); Barbour, 1937 (in part.); Mertens, 1938 (in part.); Mertens, 1939 (in part.); Cochran, 1941 (in part.); Schwartz & Thomas, 1975 (in part.); MacLean et al., 1977 (in part.); Schwartz & Henderson, 1991 (in part.); Fobes et al., 1993 (in part.); Powell et al., 1999 (in part.); Powell & Henderson, 2012 (in part.). Audantia cybotes: Nicholson et al., 2014 (in part.); Nicholson et al., 2012 (in part.); Nicholson et al., 2018 (in part.). Ctenonotus cybotes: Savage & Guyer, 1989 (in part.). Holotype. SMF 97872, an adult male from Manatí Park Bávaro (18.64735, -68.42858), 20 m, Province La Altagracia, Dominican Republic; collected 19 October 2013 by Gunther Köhler. Field tag number GK-4693. Paratypes. SMF 97870 (adult male), 97874 (adult female), same collecting data as holotype. Diagnosis. A species of the genus Audantia (our Species 2 “higuey”) that differs from all congeners by the combination of having (1) smooth ventral scales; (2) male dewlap dirty white with yellowish, greenish or orange suffusions, and with homogeneously distributed, upper portion of dewlap mostly covered by gorgetals with little free skin, and gorgetals reduced in size in central portion; (3) no dark gular streaks in males; (4) no patch of enlarged scales in nuchal region; (5) a double row of enlarged, keeled and non-mucronate vertebral scales; (6) usually two sublabial scales in contact with infralabials; (7) 166–212 scales around midbody in males; and (8) keeled scales on dorsal surfaces of upper forelimb and anterior surface of thigh. Audantia higuey differs from A. armouri by having the upper portion of dewlap mostly covered by gorgetals with little free skin and gorgetals reduced in size in central portion (vs. moderately spaced gorgetals, and all scales medium-sized on posterior half of dewlap); and by having 166–212 scales around midbody in males (vs. 118–172). Audantia higuey differs from A. breslini and A. cybotes by having smooth ventrals (vs. keeled, some individuals of A. breslini and A. cybotes with smooth ventrals); and by having gorgetals reduced in size in central portion (vs. all scales large on posterior half of dewlap). Audantia higuey differs further from A. cybotes by lacking dark gular streaks (vs. present); and by having moderately enlarged, non-mucronate vertebral scales (vs. greatly enlarged, at least three times the size of adjacent scales, mucronate vertebral scales). Audantia higuey differs from A. doris by having yellowish or orange suffusions on male dewlap (vs. no suffusions, but with an orange blotch in center of dewlap); by having enlarged, non-mucronate vertebral scales (vs. greatly enlarged, at least three times the size of adjacent scales, mucronate vertebral scales); by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent); and by having homogeneously distributed gorgetals (vs. heterogeneously distributed gorgetals with groups of cluttered scales). Audantia higuey differs from A. longitibialis by having yellowish or orange suffusions on male dewlap (vs. no suffusions); and by only having the upper portion of dewlap mostly covered by gorgetals with little free skin, and scales reduced in size in central portion of dewlap (vs. all scales widely spaced and small on posterior half of dewlap). Audantia higuey differs from A. marcanoi and A. strahmi by having a dirty white male dewlap with yellowish or orange suffusions (vs. rose-red at the edge, more orangish anteriorly and posteriorly, but purplish or even bluish toward the center in A. marcanoi, and orange with paler center in A. strahmi). Audantia higuey differs further from A. marcanoi by having a double row of abruptly enlarged vertebral scales (vs. vertebral scales gradually enlarged, not forming a regular double row). Audantia higuey differs from A. hispaniolae by lacking dark gular streaks (vs. present); and by having homogeneously distributed gorgetals (vs. heterogeneously distributed with groups of cluttered scales). Audantia higuey differs from A. ravifaux by having keeled scales on dorsal surface of upper forelimb and anterior surface of thigh (vs. smooth); by having homogeneously distributed gorgetals and the upper portion of dewlap mostly covered by scales with little free skin (vs. heterogeneously distributed gorgetals with groups of cluttered scales, widely spaced); and by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent). Audantia higuey differs from A. shrevei and A. saxatilis by having smooth ventrals (vs. keeled); and by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent). Audantia higuey differs further from A. shrevei by lacking a patch of greatly enlarged scales in nuchal region (vs. such a patch present). Audantia higuey differs further from A. saxatilis by having the upper portion of dewlap mostly covered by gorgetals with little free skin and gorgetals reduced in size in central portion (vs. widely spaced, more scales uncovered than covered by gorgetals; aall scales small on posterior half of dewlap). For differences between A. higuey and the species described below, see the respective accounts of the new species. Description of the holotype. Adult male (Figs. 35–36), as indicated by well-developed dewlap, and presence of a pair of greatly enlarged postcloacal scales; SVL 53.5 mm; tail length 51.0 mm (incomplete); tail distinctly compressed in cross section, tail height 3.7 mm and width 2.3 mm; axilla to groin distance 18.0 mm; head length 16.8 mm, head length/SVL ratio 0.31; snout length 7.4 mm; head width 9.7 mm; longest toe of adpressed hind limb reaching to level of anterior margin of eye; shank length 17.1 mm, shank length/head length ratio 1.02; longest finger of extended forelimb reaching to tip of snout; longest finger of adpressed forelimb reaching to 5 mm past anterior insertion of hind limbs. Dorsal head scales smooth or rugose, except weakly keeled scales on snout and supraoculars; 7 postrostrals; 6 scales between nasals; 1 elongate prenasal scale on each side, distinct from circumnasal and in contact with both rostral and first supralabial; circumnasal separated from first supralabial by one scale; scales in deep prefrontal depression smooth or rugose; supraorbital semicircles well-developed, broadly in contact medially; supraorbital disc composed of 4 to 5 moderately enlarged, keeled scales arranged in three rows; circumorbital row complete, therefore, enlarged supraorbital scales separated from supraorbital semicircles; a very large elongated superciliary, followed posteriorly by a much smaller, overlapping one and by several small, keeled scales; 2–4 rows of small keeled scales extending between enlarged supraorbitals and large superciliary; a deep parietal depression present; interparietal scale well-developed, 2.5 x 1.6 mm (length x width), surrounded by scales of moderate size; 2 scales present between interparietal and supraorbital semicircles; canthal ridge distinct, composed of 3 large and 1 small anterior canthal scales; 8 scales present between second canthals; 8 scales present between posterior canthals; 49 (right)–48 (left) mostly keeled loreal scales in a maximum of 7 (right)–8 (left) horizontal rows; 8 keeled subocular scales arranged in a single row; 6 supralabials to level below center of eye; suboculars separated from supralabials by a complete scale row; ear opening 1.4 x 2.0 mm (length x height); mental distinctly wider than long, almost completely divided medially, bordered posteriorly by 7 postmentals, outer ones much larger than median ones; 6 infralabials to level below center of eye; sublabials greatly enlarged (< four times the size of medial postmental scales), one in contact with infralabials; scales in sublabial row much larger than scales medially adjacent to this row; pointed granular scales present on chin and throat; dewlap large, extending from level below anterior margin of eye onto chest; gorgetals homogeneously, those in central portion reduced in size; a nuchal crest and a dorsal ridge present; dorsum of body with keeled, granular scales; 2 medial rows distinctly enlarged, mostly more than twice the size of adjacent body scales; largest dorsal scales about 0.35 x 0.30 mm (length x width); about 50 medial dorsal scales in one head length; about 67 medial dorsal scales between levels of axilla and groin; lateral scales keeled, granular and more or less homogeneous in size, average size 0.12 mm in diameter; ventrals at midbody smooth, flat, almost cycloid, imbricate, about 0.65 x 0.75 mm (length x width); about 40 medial ventral scales in one head length; about 43 medial ventral scales between levels of axilla and groin; 212 scales around midbody; all caudal scales keeled; middorsal caudal scales distinctly enlarged, forming a low crest; lateral caudal scales without whorls of enlarged scales, although an indistinct division in segments is discernible; a pair of greatly enlarged postcloacal scales present, about 1.6 mm wide; no tube-like axillary pocket present; scales on dorsal surface of upper forelimb strongly keeled, mucronate, imbricate; scales on anterior surface of thigh strongly keeled, mucronate, imbricate; digital pads dilated, dilated pad three times the width of non-dilated distal phalanx; distal phalanx narrower than and raised from dilated pad; 31 lamellae under Phalanges II–IV of Toe IV of hind limbs; 10 scales under distal phalanx of Toe IV of hind limbs. The completely everted hemipenis (Fig. 37) is a medium-sized, slightly bilobate organ; sulcus spermaticus bordered by well-developed sulcal lips and opening into a single large apical field void of ornamentation; an asulcate finger-like processus present; apex strongly calyculate, truncus with transverse folds. The coloration in life was recorded as follows: dorsal surface Beige (254) with Warm Sepia (40) chevrons on dorsal body; lateral side of body Drab-Gray (256) with a Cyan White (156) longitudinal stripe bordered by Mahogany Red (34) flecks; dorsal surface of limbs Verona Brown (37) with ill-defined Medium Neutral Gray (298) bands; edge of eye lid Pale Horn Color (11); iris Parrot Green (121); dewlap Pale Greenish White (97) with Light Yellow Ocher (13) suffusions; ventral surface of head Smoky White (261); ventral surface of body Pale Buff (1). Coloration after almost five years preservation in 70 % ethanol was recorded as follows: Dorsal ground color Smoke Gray (267) with Vandyke Brown (281) chevrons; ventral surface of head Cream White (52) with Pale Neutral Gray (296) stipples; ventral surface of body Smoky White (261); ventral surfaces of legs Light Buff (2); ventral surfaces of fingers and toes Dark Brownish Olive (127); surfaces of tail Pale Buff (1) ventrally and Pale Neutral Gray (296) dorsally. Variation. The paratypes agree well with the holotype in general appearance; morphometrics and scalation (see Table II). In most individuals the longest toe of adpressed hind leg reaching to a point between anterior and posterior level of eye, exceptionally to a point between eye and nostril.
The coloration in life of an adult female (MNHNSD 23.3599) was recorded as follows: dorsum of head Drab (19); lateral side of body Beige (254) with Medium Plumbeous (294) suffusions posteriorly, and with a Tawny Olive (17) longitudinal stripe and an ill-defined Pratt’s Payne’s Gray (293) stripe edged by Sepia (279) flecks; dorsal surface of limbs Walnut Brown (27) with indistinct Maroon (39) crossbars; edge of eye lid Light Chrome Orange (76); iris Olive-Green (124); Sepia (279) streaks radiating out from eye; ventral surface of head Smoky White (261) with irregular Olive-Brown (278) streaks; ventral surface of body Light Buff (2). Etymology. The species name (higuey) is a noun in apposition referring to the distribution of the species in eastern Hispaniola, the region of the former Taino kingdom of Higüey. Geographic distribution. As currently known, Audantia higuey is distributed across the eastern portion of the Dominican Republic, from near sea level to 290 m a. s. l. (Fig. 18). Natural history notes. As typical for most species of this genus, Audantia higuey is found in a wide range of environments, and often in disturbed habitats (Fig. 40). There these lizards can be observed perching head-down on bushes and trees. Sleeps at night on leafs of bushes. Henderson & Powell (2009) provided a summary of the natural history of “Anolis cybotes” which under our concept is represented by several species. Conservation. Given its geographic range and capability to live in anthropogenically disturbed habitats, we consider the conservation status of Audantia higuey to be Least Concern based on the IUCN Red List Categories and Criteria (IUCN, 2012). Audantia australis sp. nov. Southern Stout Anole ZOOBANK urn:lsid:zoobank.org: act:95668B57-FEB7-465F-A34E-9465545C5362. Figs. 41–43 Anolis cybotes: Boulenger, 1885 (in part.); Schmidt, 1921 (in part.); Cochran, 1928 (in part.); Barbour & Loveridge, 1929 (in part.); Barbour, 1930a (in part.); Barbour, 1930b (in part.); Schwartz & Thomas, 1975 (in part.); Schwartz, 1979 (in part.); Wyles & Gorman, 1980 (in part.); Schwartz, 1980 (in part.); Henderson et al., 1984 (in part.); Henderson & Schwartz, 1984 (in part.); Schwartz, 1989 (in part.); Burnell & Hedges, 1990 (in part.); Powell et al., 1996 (in part.); Queiroz et al., 1998 (in part.); Powell et al., 1999 (in part.); Poe, 2004 (in part.); Nicholson et al., 2005 (in part.); Henderson & Powell, 2009 (in part.); Boistel et al., 2011 (in part.); Kolbe et al., 2011 (in part.); Poe, 2013 (in part.); Köhler, 2014 (in part.); Muñoz et al., 2014a (in part.); Klaczko et al., 2015 (in part.); Giovannotti et al., 2017 (in part.); Poe et al., 2017 (in part.). Anolis cybotes cybotes: Cochran, 1934 (in part.); Barbour, 1935 (in part.); Barbour, 1937 (in part.); Cochran, 1941 (in part.); Schwartz & Thomas, 1975 (in part.); MacLean et al., 1977 (in part.); Schwartz & Henderson, 1991 (in part.); Fobes et al., 1993 (in part.); Powell et al., 1999 (in part.); Powell & Henderson, 2012 (in part.). Audantia cybotes: Nicholson et al., 2014 (in part.); Nicholson et al., 2012 (in part.); Nicholson et al., 2018 (in part.). Ctenonotus cybotes: Savage & Guyer, 1989 (in part.). Holotype. SMF 104272, an adult male from 9.4 km S of Aceitillar (26.6 km N of Cabo Rojo) (18.1083, -71.6200), 710 m, Province Pedernales, Dominican Republic; collected 26 July 1991 by S. Blair Hedges, Nicholas Plummer, and Richard Thomas. Field tag number SBH-192576. Paratypes. SMF 104273, USNM 575285–86, same collecting data as holotype; SMF 104274-75, USNM 575287, from 18.2 km N. Pedernales at stream (Los Arroyos border road) (18.155; -71.75), 200 m, Province Pedernales, Dominican Republic; collected 20 August 2015 by S. Blair Hedges, Matthew Heinicke, and Nicolás Corona; SMF 104276, near Altagracia (18.19389; -71.73306), 670 m, Province Pedernales, Dominican Republic; collected 20 August 2015 by S. Blair Hedges, Matthew Heinicke, and Nicolás Corona; SMF 104277, Mencía-Altagracia road, 1 km S Altagracia (18.1781; -71.72965), 700 m, collected 20 August 2015 by S. Blair Hedges, Matthew Heinicke, and Nicolás Corona. All paratypes are adult males except SMF 104274–75, USNM 575287, 575285 that are adult females, and USNM 575286 in which the sex was not determined. Diagnosis. A species of the genus Audantia (our Species 3 “australis”) that differs from all congeners by the combination of having (1) smooth ventral scales; (2) male dewlap dirty white with yellowish or orange suffusions, and with homogeneously distributed gorgetal scales, reduced in size in central portion of dewlap; (3) dark gular streaks present in males; (4) no patch of enlarged scales in nuchal region; (5) a double row of weakly enlarged, usually less than twice the size of adjacent scales, non-mucronate vertebral scales; (6) usually one sublabial scale in contact with infralabials; (7) 206–240 scales around midbody in males; and (8) keeled scales on dorsal surfaces of upper forelimb and anterior surface of thigh. Audantia australis differs from A. armouri, A. breslini, A. shrevei, and A. saxatilis by having dark gular streaks (usually absent); and by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent). Audantia australis differs further from A. armouri by having 206–240 scales around midbody in males (vs. 118–172). Audantia australis differs further from A. shrevei by lacking a patch of greatly enlarged scales in nuchal region (vs. such a patch present). Audantia australis differs from A. cybotes by having smooth ventral scales (vs. keeled, smooth in some individuals); by having a double row of only weakly enlarged, usually less than twice the size of adjacent scales, non-mucronate vertebral scales (vs. greatly enlarged, at least three times the size of adjacent scales, and mucronate); by having a dirty white male dewlap with yellowish or orange suffusions (vs. suffusions absent); and by having smaller gorgetal scales in central region of dewlap (vs. all large). Audantia australis differs from A. doris by having dark gular streaks (usually absent); by having a male dewlap with homogeneously distributed, narrowly spaced gorgetal scales (vs. heterogeneously distributed with groups of cluttered scales); and by having 206–240 scales around midbody in males (vs. 166–184). Audantia australis differs from A. marcanoi and A. strahmi by having a dirty white male dewlap with yellowish or orange suffusions (vs. rose-red at the edge, more orangish anteriorly and posteriorly, but purplish or even bluish toward the center in A. marcanoi, and orange with paler center in A. strahmi). Audantia australis differs further from A. marcanoi by having a well-defined double row of enlarged vertebral scales (vs. vertebral scales gradually enlarged, not forming a regular double row). Audantia australis differs from A. hispaniolae and A. longitibialis by having a male dewlap with homogeneously distributed gorgetal scales, all large on posterior half of dewlap (vs. heterogeneously distributed, somewhat spaced, and with groups of cluttered scales, scales smaller in central region of dewlap or all gorgetals small). Audantia australis differs further from A. hispaniolae by having 180–222 scales around midbody in males (vs. 214–244); and by lacking yellowish or orange suffusions on male dewlap (vs. often present). Audantia australis differs from A. higuey by having dark gular streaks (vs. absent); by having a weakly keeled double row of only weakly enlarged, usually less than twice the size of adjacent scales vertebrals scales (vs. strongly keeled and greatly enlarged, at least three times the size of adjacent scales); and by having 206–240 scales around midbody in males (vs. 166–212). Audantia australis differs from A. ravifaux by having keeled scales on dorsal surface of upper forelimb and anterior surface of thigh (vs. smooth); by having homogeneously distributed gorgetals and the upper portion of dewlap mostly covered by scales with little free skin (vs. heterogeneously distributed gorgetals with groups of cluttered scales, widely spaced); and by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent). For differences between A. australis and the species described below, see the respective accounts of the new species. Description of the holotype. Adult male (Figs. 41–42), as indicated by well-developed dewlap, and presence of a pair of greatly enlarged postcloacal scales; SVL 69.0 mm; tail length 128.0 mm (complete); tail distinctly compressed in cross section, tail height 4.2 mm and width 2.5 mm; axilla to groin distance 26.6 mm; head length 21.3 mm, head length/SVL ratio 0.31; snout length 9.2 mm; head width 12.3 mm; longest toe of adpressed hind limb reaching to level of anterior margin of eye; shank length 20.2 mm, shank length/head length ratio 0.95; longest finger of extended forelimb reaching to tip of snout; longest finger of adpressed forelimb reaching to 5 mm past anterior insertion of hind limbs. Dorsal head scales smooth or rugose, except weakly keeled scales on snout and supraoculars; 7 postrostrals; 6 scales between nasals; 1 elongate prenasal scale on each side, distinct from circumnasal and in contact with both rostral and first supralabial; circumnasal separated from first supralabial by one scale; scales in deep prefrontal depression smooth or rugose; supraorbital semicircles well-developed, narrowly in contact medially; supraorbital disc composed of 2 to 3 moderately enlarged, keeled scales arranged in three rows; circumorbital row complete, therefore, enlarged supraorbital scales separated from supraorbital semicircles; a very large elongated superciliary, followed posteriorly by a much smaller, overlapping one and by several small, keeled scales; three rows of small keeled scales extending between enlarged supraorbitals and large superciliary; a deep parietal depression present; interparietal scale well-developed, 2.9 x 1.4 mm (length x width), surrounded by scales of moderate size; 2 scales present between interparietal and supraorbital semicircles; canthal ridge distinct, composed of 3 large and 3 small anterior canthal scales; 8 scales present between second canthals; 12 scales present between posterior canthals; 53 (right)–57 (left) mostly keeled loreal scales in a maximum of 7(right)–8 (left) horizontal rows; 7 to 8 keeled subocular scales arranged in a single row; 6 supralabials to level below center of eye; suboculars separated from supralabials by a complete scale row; ear opening 2.0 x 2.6 mm (length x height); mental distinctly wider than long, almost completely divided medially, bordered posteriorly by 6 postmentals, outer ones much larger than median ones; 6 infralabials to level below center of eye; sublabials greatly enlarged (< four times the size of medial postmental scales), one in contact with infralabials; scales in sublabial row much larger than scales medially adjacent to this row; pointed granular scales present on chin and throat; dewlap large, extending from level below anterior margin of eye onto chest; gorgetals heterogeneously distributed with groups of cluttered scales, those in central portion reduced in size; a nuchal crest and a dorsal ridge present; dorsum of body with keeled, granular scales; 2 medial rows distinctly enlarged, mostly more than twice the size of adjacent body scales; largest dorsal scales about 0.35 x 0.30 mm (length x width); about 52 medial dorsal scales in one head length; about 81 medial dorsal scales between levels of axilla and groin; lateral scales keeled, granular and more or less homogeneous in size, average size 0.12 mm in diameter; ventrals at midbody smooth, flat, almost cycloid, imbricate, about 0.65 x 0.75 mm (length x width); about 42 medial ventral scales in one head length; about 56 medial ventral scales between levels of axilla and groin; 240 scales around midbody; ventral basal caudal scales smooth, all other caudal scales keeled; middorsal caudal scales distinctly enlarged, forming a low crest; lateral caudal scales with whorls of enlarged scales; a pair of greatly enlarged postcloacal scales present, about 1.7 mm wide; no tube-like axillary pocket present; scales on dorsal surface of upper forelimb strongly keeled, mucronate, imbricate; scales on anterior surface of thigh strongly keeled, mucronate, imbricate; digital pads dilated, dilated pad three times the width of non-dilated distal phalanx; distal phalanx narrower than and raised from dilated pad; 32 lamellae under Phalanges II–IV of Toe IV of hind limbs; 9 scales under distal phalanx of Toe IV of hind limbs. The coloration in life was recorded as follows: dorsum of head Cinnamon Drab (259); lateral side of body Cinnamon Drab (50) with a Pale Emerald Green (141) longitudinal stripe bordered by Mahogany Red (34) lines, and an indistinct dorsolateral Yellowish Olive-Green (118) stripe; dorsal surface of limbs Verona Brown (37) with indistinct Maroon (39) crossbars on hind limbs; edge of eye lid Whitish Lime Green (111); iris Hooker’s Green; dewlap Light Flesh Color (250) with weak Sepia (279) streaks; ventral surface of head Smoky White (261) with irregular, weak Sepia (279) streaks; ventral surface of body Light Flesh Color (250). Coloration after 27 years preservation in 70 % ethanol was recorded as follows: Dorsal ground color Hair Brown (277) with a Fuscous (283) vertebral line; ventral surface of head Smoke Gray (266); ventral surface of body Glaucous (289) with Brownish Olive (276) speckles; ventral surfaces of legs Cinnamon-Drab (50) with Prout`s Brown (47) speckles; ventral surfaces of fingers and toes Grayish Horn Color (268); dorsal, lateral and ventral surfaces of tail Raw Umber (280). Variation. The paratypes agree well with the holotype in general appearance (see also Fig. 43); morphometrics and scalation (see Table II). In most individuals the longest toe of adpressed hind leg reaching to a point between anterior and posterior level of eye, exceptionally to a point between eye and nostril or ear and eye. Etymology. The species name (australis) is an adjective meaning “southern,” referring to the distribution of the species on the southern paleo-island of Hispaniola. Geographic distribution. As currently known, Audantia australis is restricted to the central and eastern portions of the Tiburón Peninsula, Haiti and adjacent areas in the Dominican Republic south of the Sierra de Bahoruco from near sea level to 1630 m a. s. l. (Fig. 18). Natural history notes. As typical for the species in this genus, Audantia australis occurs in a wide array of habitats including secondary forest and within villages (Fig. 44). It was found sleeping at night on leaves of bushes about 1 m above ground. Conservation. Given its geographic range and capability to live in anthropogenically disturbed habitats, we consider the conservation status of Audantia australis to be Least Concern based on the IUCN Red List Categories and Criteria (IUCN, 2012). Audantia aridius sp. nov. Desert Stout Anole ZOOBANK urn:lsid:zoobank.org: act:D83E799C-483C-4A66-8F9E-AC83A9A40743. Figs. 46–48 Anolis cybotes: Boulenger, 1885 (in part.); Schmidt, 1921 (in part.); Barbour & Loveridge, 1929 (in part.); Barbour, 1930a (in part.); Barbour, 1930b (in part.); Schwartz & Thomas, 1975 (in part.); Schwartz, 1979 (in part.); Wyles & Gorman, 1980 (in part.); Schwartz, 1980 (in part.); Henderson et al., 1984 (in part.); Henderson & Schwartz, 1984 (in part.); Schwartz, 1989 (in part.); Burnell & Hedges, 1990 (in part.); Powell et al., 1996 (in part.); Queiroz et al., 1998 (in part.); Cast et al., 2000; Sifers et al., 2001 (in part.); Poe, 2004 (in part.); Nicholson et al., 2005 (in part.); Henderson & Powell, 2009 (in part.); Boistel et al., 2011 (in part.); Kolbe et al., 2011 (in part.); Poe, 2013 (in part.); Wollenberg et al., 2013 (in part.); Köhler, 2014 (in part.); Muñoz et al.,2014a (in part.); Muñoz et al., 2014b (in part.); Klaczko et al., 2015 (in part.); Conover et al., 2015 (in part.); Giovannotti et al., 2017 (in part.); Poe et al., 2017 (in part.); Boronow et al., 2018 (in part.); Kahrl et al., 2018 (in part.). Anolis cybotes cybotes: Cochran, 1934 (in part.); Barbour, 1935 (in part.); Barbour, 1937 (in part.); Mertens, 1938 (in part.); Mertens, 1939 (in part.); Cochran, 1941 (in part.); Schwartz & Thomas, 1975 (in part.); MacLean et al., 1977 (in part.); Schwartz & Henderson, 1991 (in part.); Fobes et al., 1993 (in part.); Powell & Henderson, 2012 (in part.). Audantia cybotes: Nicholson et al., 2014 (in part.); Nicholson et al., 2012 (in part.); Nicholson et al., 2018 (in part.). Ctenonotus cybotes: Savage & Guyer, 1989 (in part.) Holotype. SMF 97896, an adult male from near Cortico (18.11163, -71.22293), 1340 m, Province Barahona, Dominican Republic; collected 31 October 2013 by Gunther Köhler. Field tag number GK-4823. Paratypes. All from Barahona Province, Dominican Republic: SMF 97895, Barahona, Hotel Costa Larimar (18.199622; -71.086953), 10 m, collected 29 October 2013 by Gunther Köhler; SMF 97892–94, Los Lirios (18.11345; -71.2617), 1110 m, collected 29 October 2013 by Gunther Köhler; MNHNSD 23.3620, near Polo (18.1135; -71.26964), 855 m, collected 31 October 2013 by Gunther Köhler; SMF 104162-63, 2.9 miles NW La Ciénaga (18.07022; -71.12332), 355 m, collected 30 July 1999 by locals; USNM 329093 11.3 km S Barahona (measured from Hotel Caribe) (18.1172;-71.0717), 20 m, collected 20 August 1984 by S. Blair Hedges and Richard Thomas; USNM 329090-92, 20.8 km S Cabral (18.0964; -71.2819), 975 m, collected 19 August 1984 by S. Blair Hedges and Richard Thomas; USNM 329089, ca. 6–7 km NW Paraíso (18.0274; -71.1920), 180 m, collected 13 August 1983 by S. Blair Hedges. All paratypes are adult males except MNHNSD 23.3620 and USNM 329090 which are adult females, and USNM 329089 and 329093 in which the sex was not determined. Diagnosis. A species of the genus Audantia (our Species 4 “aridius”) that differs from all congeners by the combination of having (1) smooth ventral scales; (2) male dewlap dirty white with yellowish or orange suffusions, and with homogeneously distributed gorgetal scales, reduced in size in central portion of dewlap; (3) no dark gular streaks in males; (4) no patch of enlarged scales in nuchal region; (5) a double row of greatly enlarged, at least three times the size of adjacent scales, non-mucronate vertebral scales; (6) usually two to three sublabial scale in contact with infralabials; (7) 186–234 scales around midbody in males; and (8) keeled scales on dorsal surfaces of upper forelimb and anterior surface of thigh. Audantia aridius differs from A. armouri by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent); by having widley spaced gorgetals (vs. moderately spaced gorgetals); by having a double row of greatly enlarged vertebral scales, at least three times the size of adjacent scales (vs. those scales only weakly enlarged, usually less than twice the size of adjacent scales); and by having 184–234 scales around midbody in males (vs. 118–172). Audantia aridius differs from A. breslini and A. shrevei by having smooth ventral scales (vs. usually keeled); by having dark gular streaks (usually absent); by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent); and by having a double row of greatly enlarged vertebral scales, at least three times the size of adjacent scales (vs. those scales only weakly enlarged, usually less than twice the size of adjacent scales). Audantia aridius differs further from A. shrevei by lacking a patch of greatly enlarged scales in nuchal region (vs. such a patch present). Audantia aridius differs from A. cybotes by having smooth ventral scales (vs. keeled, some individuals of A. cybotes with smooth ventrals); by lacking dark gular streaks on male dewlap (vs. those present); by having yellowish or orange suffusions (vs. absent); by having widely spaced gorgetals and scales reduced in size in central portion of dewlap (vs. all scales narrowly spaced and large on posterior half of dewlap); and by having non-mucronate vertebral scales (vs. mucronate). Audantia aridius differs from A. doris and A. hispaniolae by having homogeneously distributed gorgetals (vs. heterogeneously distributed with groups of cluttered scales). Audantia aridius differs further from A. doris by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent); by having yellowish or orange suffusions on male dewlap (vs. no suffusions, but with an orange blotch in center of dewlap); by having 186–234 scales around midbody in males (vs. 166–184); and by having non-mucronate vertebral scales (vs. mucronate). Audantia aridius differs further from A. hispaniolae by lacking dark gular streaks (vs. present). Audantia aridius differs from A. marcanoi and A. strahmi by having a dirty white male dewlap with yellowish or orange suffusions (vs. rose-red at the edge, more orangish anteriorly and posteriorly, but purplish or even bluish toward the center in A. marcanoi, and orange with paler center in A. strahmi). Audantia aridius differs further from A. marcanoi by having a well-defined double row of enlarged vertebral scales (vs. vertebral scales gradually enlarged, not forming a regular double row). Audantia aridius differs from A. longitibialis by having a male dewlap with homogeneously distributed gorgetal scales, that are smaller in central region of dewlap (vs. heterogeneously distributed with groups of cluttered scales, all gorgetals small); and by having yellowish or orange suffusions (vs. absent). Audantia aridius differs from A. ravifaux by having keeled scales on dorsal surface of upper forelimb and anterior surface of thigh (vs. smooth); by having homogeneously distributed gorgetals (vs. heterogeneously distributed with groups of cluttered scales); by having a double row of greatly enlarged vertebral scales, at least three times the size of adjacent scales (vs. those scales only weakly enlarged, usually less than twice the size of adjacent scales); and by having one or two well-defined pale longitudinal lateral stripes, usually edged with orange or olive-green (vs. such stripes absent). Audantia aridius differs from A. saxatilis by having smooth ventral scales (vs. keeled); by having yellowish or orange suffusions (vs. absent); and by having a male dewlap with gorgetal scales, that are smaller in central region of dewlap (vs. all gorgetals small). Audantia aridius differs from A. australis and A. higuey by having widely spaced gorgetals, so more skin is more uncovered than covered by gorgetals (vs. upper portion of dewlap mostly covered by gorgetals with little free skin). Audantia aridius differs further from A. australis by lacking dark gular streaks (vs. present); and by having a double row of greatly enlarged, at least three times the size of adjacent scales, vertebral scales (vs. weakly enlarged, usually less than twice the size of adjacent scales). For differences between A. aridius and the species described below, see the respective accounts of the new species. Description of the holotype. Adult male (Figs. 46–47), as indicated by everted hemipenes, well-developed dewlap, and presence of a pair of greatly enlarged postcloacal scales; SVL 70.0 mm; tail length 78.0 mm (incomplete); tail distinctly compressed in cross section, tail height 4.5 mm and width 3.3 mm; axilla to groin distance 24.3 mm; head length 21.8 mm, head length/SVL ratio 0.31; snout length 9.2 mm; head width 12.6 mm; longest toe of adpressed hind limb reaching to a point between eye and nostril; shank length 22.2 mm, shank length/head length ratio 1.02; longest finger of extended forelimb reaching to a point 1.0 mm in front of tip of snout; longest finger of adpressed forelimb reaching to a point 6.6 mm past level of anterior insertion of hind limbs. Dorsal head scales smooth or rugose, except weakly keeled supraoculars; 6 postrostrals; 6 scales between nasals; 1 elongate prenasal scale on each side, fused with circumnasal and in contact with both rostral and first supralabial; circumnasal separated from first supralabial by one scale; scales in deep prefrontal depression smooth or rugose; supraorbital semicircles well-developed, broadly in contact medially; supraorbital disc composed of 4 to 5 moderately enlarged, keeled scales arranged in three rows; circumorbital row incomplete, therefore, some enlarged supraorbital scales in contact with supraorbital semicircles; a very large elongated superciliary, followed posteriorly by a much smaller, overlapping one and by six small, keeled scales; three rows of small keeled scales extending between enlarged supraorbitals and large superciliary; a deep parietal depression present; interparietal scale well-developed, 2.1 x 1.3 mm (length x width), T-shaped, surrounded by scales of moderate size; 2 scales present between interparietal and supraorbital semicircles; canthal ridge distinct, composed of 3 large and 3 small anterior canthal scales; 8 scales present between second canthals; 8 scales present between posterior canthals; 34 (right)–32 (left) mostly keeled loreal scales in a maximum of 6 horizontal rows; 7 to 8 keeled subocular scales arranged in a single row; 7 supralabials to level below center of eye; suboculars separated from supralabials by a complete scale row; ear opening 1.6 x 2.2 mm (length x height); mental distinctly wider than long, almost completely divided medially, bordered posteriorly by 6 postmentals, outer ones much larger than median ones; 6 (right)–7 (left) infralabials to level below center of eye; sublabials greatly enlarged (< four times the size of medial postmental scales), two in contact with infralabials; scales in sublabial row much larger than scales medially adjacent to this row; pointed granular scales present on chin and throat; dewlap large, extending from level below anterior margin of eye onto chest; dewlap more or less homogeneously covered with moderate-sized, widely spaced gorgetal scales, those in central portion reduced in size; a nuchal crest and a dorsal ridge present; dorsum of body with keeled, granular scales; 2 medial rows distinctly enlarged, more than twice the size of adjacent body scales; largest dorsal scales about 0.60 x 0.50 mm (length x width); about 48 medial dorsal scales in one head length; about 62 medial dorsal scales between levels of axilla and groin; lateral scales keeled, granular and more or less homogeneous in size, average size 0.18 mm in diameter; ventrals at midbody smooth, flat, almost cycloid, imbricate, about 0.70 x 0.70 mm (length x width); about 38 medial ventral scales in one head length; about 50 medial ventral scales between levels of axilla and groin; 220 scales around midbody; all caudal scales keeled; middorsal caudal scales distinctly enlarged, forming a low crest; lateral caudal scales with whorls of enlarged scales; a pair of greatly enlarged postcloacal scales present, about 2.0 mm wide; no tube-like axillary pocket present; scales on dorsal surface of upper forelimb strongly keeled, mucronate, imbricate; scales on anterior surface of thigh strongly keeled, mucronate, imbricate; digital pads dilated, dilated pad twice the width of non-dilated distal phalanx; distal phalanx narrower than and raised from dilated pad; 28 lamellae under Phalanges II–IV of Toe IV of hind limbs; 10 (right; missing distal phalanx left) scales under distal phalanx of Toe IV of hind limbs. Coloration after 5 years preservation in 70 % ethanol was recorded as follows: Dorsal ground color Glaucous (272) with Hair Brown (277) chevrons; ventral surface of head Pale Greenish White (97); dewlap Cream White (52) with Light Neutral Gray (297) stipples; ventral surface of body Paris White (139); ventral surfaces of legs Light Buff (2) with Beige (254) stipples; ventral surfaces of fingers and toes Glaucous (289) with Hair Brown (277) tips; surfaces of tail Pale Buff (1) ventrally and Pale Neutral (296) dorsally. Variation. The paratypes agree well with the holotype in general appearance; morphometrics and scalation (Table II). In most individuals the longest toe of adpressed hind leg reaching to a level of anterior margin of eye, exceptionally to a point between eye and nostril. The coloration in life of an adult male (SMF 97894) was recorded as follows: lateral side of body Cinnamon Drab (259) with Vinaceous (247) suffusions edging an Olive Horn Color (16) longitudinal stripe; dorsal surface of limbs Verona Brown (37) with indistinct Mars Brown (25) cross bars; edge of eye lid Cream Color (12); iris Dark Brownish Olive (127); dewlap Smoky White (261) with Light Buff (2) suffusions; ventral surface of head Smoky White (261); ventral surface of body Pale Buff (1). The completely everted hemipenis (SMF 97896; Fig. 48) is a medium-sized, slightly bilobate organ; sulcus spermaticus bordered by well-developed sulcal lips and opening into a single large apical field void of ornamentation; an asulcate ridge present; apex strongly calyculate, truncus with transverse folds. The everted hemipenis of two other specimens (SMF 97892–93) agree well with this description Etymology. The species name (aridius) is a noun in apposition derived from the Latin adjective aridus (“dry”), in allusion to the arid habitats where this species occurs. Geographic distribution. As currently known, Audantia aridius is restricted to the eastern portion of the Barahona Peninsula, Dominican Republic, from near sea level to 1340 m a. s. l. (Fig. 18). Natural history notes. The habitat near the type locality of Audantia aridius is disturbed broadleaf forest (Fig. 49). We observed and collected this species mostly perched on rocks and low vegetation during daytime. Also, it was commonly found on and under rocks, or on tree trunks low to the ground, during the day. At night these lizards can be found sleeping on branches and on the upper suface of leafs as it is typical for most anoles. Conservation. Given its geographic range and capability to live in anthropogenically disturbed habitats, we consider the conservation status of Audantia aridius to be Least Concern based on the IUCN Red List Categories and Criteria (IUCN, 2012). KEY TO THE SPECIES OF THE GENUS AUDANTIA 1a. Ventral scales keeled……………………………………………………………………….. 2 1b. Ventral scales smooth…………………………………………………………………..........5 2a. Several rows of scales in nuchal region much enlarged, same size or larger than those in vertebral double row………………………………………………………… . Audantia shrevei 2b. Only two rows of scales in nuchal region slightly enlarged, larger than adjacent scales…... 3 3a. All gorgetals on posterior portion of male dewlap large, only slightly reduced towards base, all narrowly spaced…………………………………………………... Audantia cybotes (in part) 3b. All gorgetals on male dewlap small and widely spaced…………………………………….. 4 4a. Neck and dorsum with clearly defined blackish, very dark gray, or dark brown crossbands; SVL in males to 67 mm, in females to 54 mm………………………………Audantia saxatilis 4b. Dorsal crossbands blurred to absent, never clearly delineated, usually no dark band on neck; SVL in males to 60 mm, in females to 45 mm…………………………………Audantia breslini 5a. Male dewlap red or orange in life…………………………………………………………….6 5b. Male dewlap white or pale gray in life, with or without suffusions of yellow, green, or orange………………………………………………………………………………...................7 6a. A double row of distinctly and abruply enlarged vertebral scales………….. Audantia strahmi 6b. Scales in vertebral area gradually and slightly enlarged, not forming a distinct double row………………………………………………………………………….Audantia marcanoi 7a. Habitus very stout; usually <170 scales around midbody; restricted to the highlands of the Sierra de Bahoruco, Dominican Republic, and Massif de La Selle, Haiti………Audantia armouri 7b. Habitus not particularly stout; usually >170 scales around midbody; distributed in the lowlands of Hispaniola…………………………………………………………………………………… 8 8a. Scales on anterior surface of thigh greatly enlarged and smooth……………Audantia ravifaux 8b. Scales on anterior surface of thigh only slightly enlarged and usually keeled……………… 9 9a. All gorgetals on posterior portion of male dewlap large, only slightly reduced towards base, all narrowly spaced………………………………………………… . .Audantia cybotes (in part) 9b. Gorgetals in central portion of male dewlap much reduced in size and widely spaced, or all gorgetals small and widely spaced………………………………………………………………10 10a. Gorgetals on male dewlap heterogeneously distributed with groups of cluttered scales…… 11 10b. Gorgetals on male dewlap more or less homogeneously distributed………………………..12 11a. Gorgetals on male dewlap in central portion of male dewlap much reduced in size; dark streaks present on anterior base of male dewlap……………………………Audantia hispaniolae 11b. All gorgetals on male dewlap small and widely spaced; no dark streaks on anterior base of male dewlap…………………………………………………………….Audantia longitibialis 12a. Male dewlap with a central orange blotch in life; usually <185 scales around midbody; restricted to Île de la Gonâve, Haiti…………………………………………….....Audantia doris 12b. Male dewlap without a central orange blotch in life; usually >185 scales around midbody; distributed on the main island of Hispaniola……………………………………………………13 13a. Usually one enlarged sublabial scale in contact with infralabials; dark streaks present on anterior base of male dewlap; scales in vertebral double row weakly enlarged, about twice the size of adjacent scales……………………………………………………….Audantia australis 13b. Usually two or three enlarged sublabial scales in contact with infralabials; no dark streaks on anterior base of male dewlap; scales in vertebral double row greatly enlarged, more than twice the size of adjacent scales………………………………………………………………………………14 14a. 214–270 scales around midbody in males; all gorgetals widely spaced, more skin uncovered than covered by scales; hemipenis with an asulcate ridge………………………Audantia aridius 14b. 166–212 scales around midbody in males; upper portion of male dewlap mostly covered by gorgetals with little free skin; hemipenis with an asulcate finger-like processus….……………………………………………………………………Audantia higuey This study shows more diversity among the anoles of the genus Audantia of Hispaniola than reflected in the current literature. In this study, we recognize 14 species—four of which we describe as new species—and it is likely that more, undescribed, species are present. Table IV summarizes the taxonomic changes as a result of this study. Our phylogenetic tree (Fig. 1) shows that there is considerable genetic divergence among geographically isolated populations, indicating a lack of or reduced gene flow. Some of the species we recognize (e.g., A. aridius, A. armouri, A. australis, A. higuey, and A. ravifaux) exhibit considerable genetic structure, to a point that most of these subclades qualify as candidate species sensu Vieites et al. (2009). To test whether these 19 candidate species could be differentiated in external morphology, we scored the available specimens for a set of 39 characters of scalation and morphometrics. However, no prominent differentiation in morphology could be uncovered within the larger clades that we recognize as species level units, and therefore we tentatively consider the respective genetic subclades to be conspecific. More field and lab studies are needed in order to test the hypothesis that these diverging allopatric populations indeed are conspecific. Species delimitation methods using molecular data often generate a large number of putative (undescribed) species. However, many of the putative species so delimited are simply isolated populations that do not represent biological species (Sukumaran & Knowles, 2017). For this reason, and because our study includes a more comprehensive (systematic) delimitation of species, considering molecular, morphological, and geographic information, we did not use molecular (only) delimitation methods. At first glance, many of the species of the genus Audantia are not easy to differentiate and depict a high degree of visual resemblance, mostly being morphologically conservative to a point that they can be called cryptic species. Glor et al. (2003:2393) had called this phenomenon in this group of lizards “Morphological evolutionary stasis”. Nonetheless, a detailed morphological analysis allows uncovering distinct although subtle morphological differences among the species of Audantia, the differentiating characters varying between species pairs. The clade with specimens from the extreme western portion of the Tiburón Peninsula, Haiti, is assigned to Audantia cybotes sensu stricto, since the type locality of A. cybotes is “Western Hayti; from near Jeremie”. The specimens from the sister-clade could not only be differentiated by the genetic divergence shown in the phylogenetic tree, but also by morphology: For instance, specimens of A. cybotes from the western portion of the Tiburón Peninsula have keeled ventral scales, a double row of greatly enlarged mucronate vertebral scales and a male dewlap with no suffusions whereas the specimens from the central and eastern Tiburón Peninsula have smooth ventrals, only weakly enlarged non-mucronate vertebrals and a male dewlap with yellowish or orange suffusions. Therefore, the latter populations is recognized as a distinct species, A. australis. The two species are sympatric at two locations, Morne Grand Bois and Morne Bois Pangnol, where they may be hybridizing (see below). In the case of A. higuey and A. hispaniolae, the DFAs (Figs. 4-5) show no overlapping polygons, indicating a high degree of differentiation in the morphological characters that were compared. Audantia hispaniolae and A. higuey are widely sympatric across the eastern portion of the Dominican Republic. Some of the recognized species are more readily differentiated in morphology, such as A. ravifaux (e.g., having smooth enlarged scales on anterior surface of thigh). We examined all extant primary types of all nominal taxa previously recognized as synonyms of Anolis cybotes Cope, 1863. As mentioned in the introduction, the type material of Audantia heatianus is in poor condition. Nevertheless, these three specimens mostly resemble A. cybotes and given its type locality “Tiburon, Hayti” (Garman, 1887), A. heatianus is assigned to the synonymy of A. cybotes. Our genetic and morphological data support the recognition of ravifaux and doris as distinct species and therefore these two former subspecies of A. cybotes were elevated to species level. Barbour (1914) recognized A. citrinellus as a valid “beautiful little species, confined to Haiti,” citing MCZ 1326 as a representative of this species. The online database of MCZ indicates that this specimen (from “Port au Prince, Hayti”) was reidentified as “Anolis cristatellus cristatellus”. In his list of the anoles “known to occur on the Neotropical islands” Barbour (1930b) states that the “type in Brit. Mus. ... is a young A. cybotes”. The nominal species A. citrinellus has remained in the synonymy of A. cybotes ever since (e.g., Cochran, 1934; Schwartz, 1989). However, the examination of the holotype of A. citrinellus clearly revealed that in external morphology it has the characteristics of Ctenonotus distichus rather than of any species of the genus Audantia. Most of the species treated in this work are distributed across the lowlands and but some reach the mid elevations of the Hispaniolan mountains. In contrast, A. armouri and A. shrevei occur primary in the highlands above 1650 masl. They both “occupy high-altitude pine forests in the Sierra de Bahoruco and Cordillera Central, respectively” (Schwartz & Henderson, 1991). Specimens assigned to A. shrevei are easily identified by having a patch of enlarged nuchal scales (Cochran, 1939), a morphological characteristic not found in the other species. Also supported by our genetic data, both nominal taxa are therefore confirmed as distinct species. Furthermore, our genetic data support the recognition of the nominal taxa A. breslini, A. longitibialis, A. marcanoi, A. strahmi, and A. saxatilis as distinct species. Some aspects of the evolutionary history of the genus Audantia are revealed by the relationships (Fig. 1), distribution (Fig. 18), and habitat preferences of the species. Six of the 14 species are endemic to the North Island: A. breslini, A. higuey, A. marcanoi, A. ravifaux, A. saxatilis, and A. shrevei. We would add a seventh species, A. hispaniolae, to that list because of its broad distribution across the North Island, even though it encroaches the South Island in a portion of the Massif de la Selle. It also occurs on Gonave, an island that is intermediate between the North and South islands of Hispaniola and has received faunal elements from both areas (Schwartz, 1978). Six species are endemic to the South Island: A. aridius, A. armouri, A. australis, A. cybotes, A. longitibialis, and A. strahmi. Audantia doris is endemic to Gonave. The North Island is older (late Eocene), in terms of the earliest exposed land (Iturralde-Vinent & MacPhee, 1999), than the South Island (late Miocene, ~10 mya), and the most basal branching species (A. marcanoi) occurs on the North Island. During the evolution of the genus, there were three invasions of the South Island: (1) The longitibialis species group (A. longitibialis and A. strahmi), the cybotes species group (A. aridius, A. australis, and A. cybotes), and A. armouri. Although we did not construct a timetree, it can be inferred that the crown ages (basal node times) of the two endemic South Island clades of multiple species (cybotes and longitibialis species groups) are younger than 10 mya. This infers a Miocene/ Pliocene evolution of the genus, in contrast to a previous estimate (Nicholson et al., 2012) of an early Eocene (54 mya) crown age, and also younger than estimates in Poe et al., (2017). In terms of habitat preferences, there is a trend in that the earliest-diverging species (A. aridius, A. breslini, A. longitibialis, A. marcanoi, A. saxatilis, A. strahmi) are xerophilic and the later-diverging species are mesophilic. This trend could be explained by global climate change during the evolution of Audantia, starting with late Miocene cooling (and drying) followed by Pliocene warming. Although hybridization among anoles is rather rare (Losos, 2009), it is a possibility in areas where closely related species are sympatric (and possibly syntopic). For the species of Audantia, this phenomenon of hybridization is most likely for the species pairs A. australis / A. cybotes, A. doris / A. hispaniolae, A. higuey / A. hispaniolae, and A. higuey / A. ravifaux. Through alterations of distribution of the respective species and/or anthropogenic habitat changes (e.g., habitat fragmentation, land use change, and displacement of species), (Allendorf et al., 2001) but also abiotic (e.g., climatic) effects, secondary contact zones and areas of sympatry may occur. In those areas of contact, hybridization can occur (Harrison, 1993). In the case of A. australis / A. cybotes overlapping polygons in the DFAs indicate specimens with intermediate characters in this group (Figs. 6–7). Also, the genetically assigned A. cybotes occurring sympatrically with A. australis have smooth ventrals (like A. australis), whereas the allopatric A. cybotes have strongly keeled ventral scales. These observations possibly indicate genetic introgression, which could be studied in the future with methods that sample nuclear genes. The hypothesis of hybridization between the other named species could not be supported by the evaluated data. Further research is needed to confirm hybridization in some of these species pairs. The large amount of deforestation in Hispaniola, and especially in Haiti (Hedges et al., 2018), has greatly disrupted habitats and might provide an explanation for hybridization, if it exists. Finally, since we have taken the liberty to suggest common names for the seven species of Audantia treated in detail in this work, we would also like to point out common names for the remaining species of this genus, already coined (Hedges et al., 2019): Audantia armouri (Black-throated Stout Anole), A. breslini (Northwestern Stout Anole; modified), A. longitibialis (Barahona Stout Anole), A. marcanoi (Red-fanned Stout Anole), A. saxatilis (Banded Stout Anole), A. shrevei (Cordillera Central Stout Anole), and A. strahmi (Bahoruco Stout Anole). ZooBank registration. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the International Commission on Zoological Nomenclature (ICZN). The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org. The LSID for this publication is as follows: urn:lsid:zoobank.org:pub:63E5E7E8-754C-45C7-B34A-36A262C2C7C2. Collecting and exportation permits to GK were issued by Bautista Roja Gómez and José ML. Mateo Felíz, Ministerio de Medio Ambiente y Recursos Naturales, Santo Domingo, Dominican Republic. For the loan of and/or access and/or providing information on specimens, we thank Patrick Campbell, The Natural History Museum (BMNH), London; James Hanken, Jonathan Losos, and José P. Rosado, Museum of Comparative Zoology, Harvard University (MCZ), Cambridge; Cristian Marte and Eveling Gabot, Museo Nacional de Historia Natural “Prof. Eugenio de Jesús Marcano” (MNHNSD), Santo Domingo, Dominican Republic; Peter Rask Møller and Daniel Klingberg Johansson, Natural History Museum of Denmark, University of Copenhagen, Zoological Museum (NHMD), Copenhagen; Jeremy Jacobs, Esther Langan, Ron W. McDiarmid, Robert Wilson, W. Ronald Heyer, and Addison Wynn, National Museum of Natural History (USNM), Washington, D.C. We thank Laura Bruce, who completed her honors thesis work at Pennsylvania State University, under the guidance of S. Blair Hedges, for generating some of the DNA sequences used in the present work. We are grateful to Claus Bo Petersen who shared valuable information and thoughts with us about the original publications of Anolis riisei and A. cybotes, respectively. Also, Claus helped CZ during her stay in Copenhagen with logistics and assisted her during her visit to the Natural History Museum of Denmark, University of Copenhagen, Zoological Museum (NHMD). For field assistance, Gunther Köhler thanks Eladio Fernandez, Cristian Marte, Eveling Gabot and Marcos Rodríguez. S. Blair Hedges thanks many of his students, staff, and colleagues for assistance on expeditions over three decades, including Yvonne Arias, Philippe Bayard, Tiffany Cloud, Arnaud Dupuy, Eladio Fernandez, Sarah Hanson, Jessie Haspil, Matthew Heinicke, Sixto Inchaustegui, Anderson Jean, Miguel Landestoy, Manuel Leal, Allison Loveless, Einar Madsen, Carlos Martinez, Nicholas Plummer, Jennifer Pramuk, Jessica Preston, Elisabeth Rochel, Florence Sergile, Joel Timyan, Michael Tracy, and especially Richard Thomas; Laura Bruce, Matthew Heinicke, Angela Marion; and Allison Loveless and Elisabeth Rochel for laboratory assistance; and the governments of the Republic of Haiti and the Dominican Republic for collecting and export permits. This research was supported by grants from the United States National Science Foundation (8307115, 8906325, 9123556, 9525775, 9615643, and 0918891) and the Critical Ecosystems Partnership Fund (HAI/62132) to S. Blair Hedges. We thank the anonymous reviewers of the manuscript for valuable corrections and comments that improved the work. Finally, we want to thank Carlos Suriel and Sixto Inchaustegui, Editors of Novitates Caribaea, for their invaluable support in the preparation of this article for publication.
DISCUSSION
ACKNOWLEDGMENTS
LITERATURE CITED
Allendorf, F. W., R. F. Leary, P. Spruell, & J. K. Wenburg. 2001. The problems with hybrids: setting conservation guidelines. Trends in Ecology & Evolution, 16: 613–622.
Barbour, T. 1914. A contribution to the zoogeography of the West Indies with special reference to amphibians and reptiles. Memoirs of the Museum of Comparative Zoology, 44 (2): 209–359.
Barbour, T. 1925. New Neotropical lizards. Proceedings of the Biological Society of Washington, 38: 101–102.
Barbour, T. 1930a. A list of Antillean reptiles and amphibians. Zoologica (New York), 11: 61–116.
Barbour, T. 1930b. The anoles. I. The forms known to occur on the Neotropical islands. Bulletin of the Museum of Comparative Zoology, 70: 105–143.
Barbour, T. 1935. A second list of Antillean reptiles and amphibians. Zoologica (New York), 19: 77–142.
Barbour, T. 1937. Third list of Antillean reptiles and amphibians. Bulletin of the Museum of Comparative Zoology, 82: 77–166.
Barbour, T. & A. Loveridge. 1929. Typical reptiles and amphibians in the Museum of Comparative Zoology. Bulletin of the Museum of Comparative Zoology, 69: 206–360.
Boistel, R., A. Herrel, R. Lebrun, G. Daghfous, P. Tafforeau, J. B. Losos & B. Vanhooydonck. 2011. Shake rattle and roll: the bony labyrinth and aerial descent in squamates. Integrative and Comparative Biology, 51: 957–968.
Boronow, K. E., I. H. Shields & M. M. Muñoz. 2018. Parallel Behavioral Divergence with Macrohabitat in Anolis (Squamata: Dactyloidae) lizards from the Dominican Republic. Breviora, 561: 1–17.
Boulenger, G. A. 1885. Catalogue of the Lizards in the British Museum. Taylor & Francis, London, 497 pp.
Burnell, K. L. & S. B. Hedges. 1990. Relationships of West Indian Anolis (Sauria: Iguanidae): an approach using slow-evolving protein loci. Caribbean Journal of Science, 6: 7–30.
Case, S. & E. E. Williams. 1987. The cybotoid anoles and Chamaelinorops lizards (Reptilia: Iguanidae): evidence of mosaic evolution. Zoological Journal of the Linnean Society, 91: 325–341.
Cast, E. E., M. E. Gifford, K. R. Schneider, A. J. Hardwick, Parmerlee, J. S., Jr. & R. Powell. 2000. Natural history of an anoline lizard community in the Sierra de Bahoruco, Dominican Republic. Caribbean Journal of Science, 36: 258–266.
Cochran, D. M. 1928. The herpetological collections made in Haiti and its adjoiningn islands by Walter J. Eyerdam. Proceedings of the Biological Society of Washington, 41: 53–59.
Cochran, D. M. 1934. Herpetological collections made in Hispaniola by the Utowana Expedition, 1934. Occasional Papers of the Boston Society of Natural History, 8: 163–188.
Cochran, D. M. 1939. Diagnoses of three new lizards and a frog from the Dominican Republic. Proceedings of the New England Zoological Club, 18: 1–3.
Cochran, D. M. 1941. The herpetology of Hispaniola. United States National Museum Bulletin, 177: 1–398.
Conover, A. E., E. G. Cook, K. E. Boronow & M. M. Muñoz. 2015. Effects of ectoparasitism on behavioral thermoregulation in the tropical lizards Anolis cybotes (Squamata: Dactyloidae) and Anolis armouri (Squamata: Dactyloidae). Breviora, 545, 1–13.
Cope, E. D. 1861. Notes and descriptions of anoles. Proceedings of the Academy of Natural Sciences of Philadelphia, 1861: 208–215.
Cope, E. D. 1863. Contributions to Neotropical saurology. Proceedings of the Academy of Natural Sciences of Philadelphia, 14: 176–188.
Cope, E. D. 1864. Contributions to the herpetology of tropical America. Proceedings of the Academy of Natural Sciences of Philadelphia, 16: 166–181.
Etheridge, R. 1960. The relationships of the anoles (Reptilia: Sauria: Iguanidae): An interpretation based on skeletal morphology. Ph.D. Dissertation, Univ. Michigan, Ann Arbor.
Fobes, T. M., J. S. Parmerlee, Jr, & R. Powell. 1993. Anolis cybotes Cope. Catalogue of American Amphibians and Reptiles, 564: 1–5.
Garman, S. 1887. On West Indian Reptiles. Iguanidae. Bulletin of the Essex Institute, 19: 25–50.
Giovannotti, M., V. A. Trifonov, A. Paoletti, I. G. Kichigin, P. C. M. O’Brien, F. Kasai, G. Giovagnoli, B. L. Ng, P. Ruggeri, P. Nisi Cerioni, A. Splendiani, J. C. Pereira, E. Olmo, W. Rens, V. Caputo Barucchi, & M. A. Ferguson-Smith. 2017. New insights into sex chromosome evolution in anole lizards (Reptilia, Dactyloidae). Chromosoma, 126: 245–260.
Glor, R. E., J. J. Kolbe, R. Powell, A. Larson, & J. Losos. 2003. Phylogenetic analysis of ecological and morphological diversification in Hispaniolan trunk-ground anoles (Anolis cybotes group). Evolution, 57: 2383–2397.
Harrison, R. G. 1993. Hybrid Zones and the Evolutionary Process. Oxford University Press, New York, 364 pp.
Hedges, S. B. 1996. Historical biogeography of West Indian vertebrates. Annual Review of Ecology, Evolution, and Systematics, 27: 163–196.
Hedges, S. B. 2018. CaribHerp. West Indian amphibians and reptiles. Available from http://www.caribherp.org/ (Accessed 5 September, 2018).
Hedges, S. B., R. Powell, R. W. Henderson, S. Hanson, & J. C. Murphy. 2019. Definition of the Caribbean Islands biogeographic region, with checklist and recommendations for standardized common names of amphibians and reptiles. Caribbean Herpetology, 67: 1–53.
Hedges, S. B., W. B. Cohen, J. Timyan, & Z. Yang. 2018. Haiti’s biodiversity threatened by nearly complete loss of primary forest. Proceedings of the National Academy of Sciences of the United States of America, 115: 11850–11855.
Hedges, S. B., W. E. Duellman, & M. P. Heinicke. 2008. New World direct-developing frogs (Anura: Terrarana): Molecular phylogeny, classification, biogeography, and conservation. Zootaxa, 1737: 1–182.
Henderson, R. W., & A. Schwartz. 1984. A guide to the identification of the amphibians and reptiles of Hispaniola. Special Publications in Biology and Geology, 4: 1–70.
Henderson, R. W., A. Schwartz, & S. J. Incháustegui. 1984. Guía para la identificación de los anfibios y reptiles de la Hispaniola. Ser. Mon. 1, Taller, Santo Domingo, RD.
Henderson, R. W., & R. Powell. 2009. Natural History of West Indian Reptiles and Amphibians. University Press of Florida, Gainesville, USA, 520 pp.
International Union for Conservation of Nature (IUCN) 2012. IUCN Red List Categories and Criteria, Version 3.1. Second edition. IUCN Species Survival Commission, Gland, Switzerland and Cambridge, UK. accessible at https://portals.iucn.org/library/node/10315 (accessed 17 November 2018).
Iturralde-Vinent, M. A., & R. D. E. MacPhee. 1999. Paleogeography of the Caribbean region:
implications for Cenozoic biogeography. Bulletin of the American Museum of Natural History, 238: 1–95.
Kahrl, A. F., M. Brittney, K. Ivanov, C. Wollenberg Valero, & M. A. Johnson. 2018. Ecomorphological variation in three species of cybotoid anoles. Herpetologica, 74: 29–37.
Katoh, K. & D. M. Standley, 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30: 772–780.
Klaczko, J., T. Ingram, & J. Losos. 2015. Genitals evolve faster than other traits in Anolis lizards. Journal of Zoology, 295: 44–48.
Köhler, G. 2012. Color Catalogue for Field Biologists. Herpeton, Offenbach, Germany, 49 pp.
Köhler, G. 2014. Characters of external morphology used in Anolis taxonomy - Definition of terms, advice on usage, and illustrated examples. Zootaxa, 3774: 201–257.
Kolbe, J. J., L. J. Revell, B. Szekely, E. D. Brodie & J. B. Losos. 2011. Convergent evolution of phenotypic integration and its alignment with morphological diversification in Caribbean Anolis ecomorphs. Evolution, 65: 3608–3624.
Kumar, S., G. Stecher, M. Li, C. Knyaz, & K. Tamura. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35: 1547–1549.
Losos, J. B. 1985. Male aggressive behaviour in a pair of sympatric sibling species. Breviora, 484: 1–30.
Losos, J. B. 2009. Lizards in an evolutionary tree:ecology and adaptive radiation of anoles. University of California Press, Oakland, Carlifornia, 528 pp.
MacLean. W. P., R. Kellner, & H. Dennis. 1977. Island lists of West Indian amphibians and reptiles. Smithsonian Herpetological Information Service, 40: 1–147.
Mertens, R. 1938. Amphibien und Reptilien aus Santo Domingo, gesammelt von Prof Dr. H. Böker. Senckenbergiana, 20: 332–342.
Mertens, R. 1939. Herpetologische Ergebnisse einer Reise nach der Insel Hispaniola, Westindien. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 449: 1–84.
Muñoz, M. M., & J. B. Losos. 2018. Thermoregulatory behavior simultaneously promotes and forestalls evolution in a tropical lizard. The American Naturalist, 191: E15–E26.
Muñoz, M. M., M. A. Stimola, A. C. Algar, A. Conover, A. J. Rodríguez, M. A. Landestoy, G. S. Bakken, & J. B. Losos. 2014a. Evolutionary stasis and lability in thermal physiology in a group of tropical lizards. Proceedings of the Royal Society, Biological Sciences, 281: 2013–2433.
Muñoz, M. M., J. E. Wegener, & A. C. Algar. 2014b. Untangling intra- and interspecific effects on body size clines reveals divergent processes structuring convergent patterns in Anolis lizards. The American Naturalist, 184: 636–646.
Nicholson, K. E., B. I. Crother, C. Guyer, & J. M. Savage. 2012. It is time for a new classification of anoles (Squamata: Dactyloidae). Zootaxa, 3477: 1–108.
Nicholson, K. E., B. I. Crother, C. Guyer, & J. M. Savage. 2014. Anole classification. A response to Poe. Zootaxa, 3814: 109–120.
Nicholson, K. E., B. I. Crother, C. Guyer, & J. M. Savage. 2018. Translating a clade based classification into one that is valid under the international code of zoological nomenclature:
the case of the lizards of the family Dactyloidae (Order Squamata). Zootaxa, 4461: 573–586.
Nicholson, K. E., R. E. Glor, J. J. Kolbe, A. Larson, S. Blair Hedges, & J. B. Losos. 2005. Mainland colonization by island lizards. Journal of Biogeography, 32: 929–938.
Noble, G. K. 1923a. Four new lizards from Beata Island, Dominican Republic. American Museum Novitates, 64: 1–5.
Noble, G. K. 1923b. In pursuit of the giant tree frog. Natural History, 23: 105–116.
Olson, R. E. 1990. Herpetological observations on Tiburon Peninsula, Haiti, West Indies. Bulletin of the Maryland Herpetological Society, 26: 135–152.
Poe, S. 2004. Phylogeny of anoles. Herpetological Monographs, 18: 37–89.
Poe, S. 2013. 1986 Redux: New genera of anoles (Squamata: Dactyloidae) are unwarranted. Zootaxa, 3626: 295–299.
Poe, S., A. Nieto-Montes de Oca, O. Torres-Carvajal, K. de Queiroz, J. A. Velasco, B. Truett, L. N. Gray, M. J. Ryan, G. Köhler, F. Ayala-Varela, & I. Latella. 2017. A phylogenetic, biogeographic, and taxonomic study of all extant species of Anolis (Squamata; Iguanidae). Systematic Biology, 66: 663–697.
Powell, R., & R. W. Henderson. 2012. Island lists of West Indian amphibians and reptiles. Univ. of Florida, Gainesville, Fla., 86166 pp.
Powell, R., R. W. Henderson, K. Adler, & H. A. Dundee. 1996. An annotated checklist of West Indian amphibians and reptiles. In: Powell, R. & Henderson, R. (Eds.). Contributions to West Indian Herpetology: a Tribute to Albert Schwartz. Society for the Study of Amphibians and Reptiles, Ithaca, New York, 51–93 pp.
Powell, R., J. A. Ottenwalder, & S. J. Incháustegui. 1999. The Hispaniolan Herpetofauna. Diversity, Endemism, and Historical Perspectives, with comments on Navassa Island. In: Crother, B. (Ed.). Caribbean amphibians and reptiles. Academic Press, San Diego, pp. 93–168.
Queiroz, K. de, L.-R. Chu, & J. B. Losos. 1998. A second Anolis lizard in Dominican Amber and the systematics and ecological morphology of Dominican Amber anoles. American Museum Novitates, 3249: 1–23.
Rand, A. S. 1962. Notes on Hispaniolan herpetology 5. The natural history of three sympatric species of Anolis. Breviora, 154: 1–15.
Reinhardt, J., & C. F. Lütken. 1863. Bidrag til det vestindiske Öriges og navnligen til de danskvestindiske Öers Herpetologie. Videnskabernes Meddelingen Naturhistorik Forening Kjöbenhavn, 24: 153–291.
Sabaj, M. H. 2016. Standard Symbolic Codes for Institutional Resource Collections in Herpetology and Ichthyology Version 6.5. Available from http://www.asih.org/sites/default/ files/documents/symbolic_codes_for_collections_v6.5.pdf. Accessed 30 November 2018.
Savage, J. M. & C. Guyer. 1989. Infrageneric classification and species composition of the anole genera, Anolis, Ctenonotus, Dactyloa, Norops, and Semiurus (Sauria: Iguanidae). Amphibia-Reptilia, 10: 105–116.
Schmidt, K. P. 1921. Notes on the herpetology of Santo Domingo. Bulletin of the American Museum of Natural History, 44: 7–20.
Schwartz, A. 1978. Some aspects of the herpetogeography of the West Indies. Academy of Natural Sciences, Philadelphia, Special Publication, 13: 31–51.
Schwartz, A. 1979. A new species of cybotoid anole (Sauria, Iguanidae) from Hispaniola. Breviora, 451: 1–27.
Schwartz, A. 1980. Variation in Hispaniolan Anolis whitemani Williams. Journal of Herpetology, 14: 399–406.
Schwartz, A. 1989. A review of the cybotoid anoles (Reptilia: Sauria: Iguanidae) from Hispaniola. Milwaukee Public Museum Contributions in Biology and Geology, 78: 1–32.
Schwartz, A. & R. W. Henderson. 1982. Anolis cybotes (Reptilia, Inguanidae). The eastern Hispaniolan populations. Milwaukee Public Museum Press, Milwaukee, 8 pp.
Schwartz, A. & R. W. Henderson. 1988. West Indian amphibians and reptiles. A check-list, Milwaukee Public Museum Contributions in Biology and Geology, 74: 1–264.
Schwartz, A. & R. W. Henderson. 1991. Amphibians and reptiles of the West Indies. Descriptions, distributions, and natural history. University of Florida Press, Gainesville, USA, 720 pp.
Schwartz, A. & R. Thomas. 1975. A check-list of West Indian amphibians and reptiles. Carnegie Museum of Natural History Special Publication, 1: 1-216.
Sifers, S. M., M. L. Yeska, Y. M. Ramos, R. Powell, & J. S. Parmerlee, jr, 2001. Anolis lizards restricted to altered edge habitats in a Hispaniolan cloud forest. Caribbean Journal of Science, 37: 55–62.
Stamatakis, A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England), 30: 1312–1313.
Sukumaran, J. & L. L. Knowles. 2017. Multispecies coalescent delimits structure, not species. Proceedings of the National Academy of Sciences, 114: 1607–1612.
Uetz P., P. Freed, & J. Hošek, (eds.) 2019. The Reptile Database, http://www.reptile-database. org, (Accessed 22 November 2018).
Vieites, D. R., K. C. Wollenberg, F. Andreone, J. Köhler, F. Glaw, & M. Vences. 2009. Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proceedings of the National Academy of Sciences of the United States of America, 106: 8267–8272.
Williams, E. E. 1963. Anolis whitemani, new species from Hispaniola (Sauria, Iguanidae). Breviora, 197: 1–8.
Williams, E. E. 1975. Anolis marcanoi new species: sibling to Anolis cybotes: description and field evidence. Breviora, 430: 1–9.
Williams, E. E. 1976. West Indian anoles. A taxonomic and evolutionary summary. 1. Introduction and a species list. Breviora, 440: 1–21.
Wollenberg, K. C., I. J. Wang, R. E. Glor, & J. B. Losos. 2013. Determinism in the diversification of Hispaniolan trunk-ground anoles (Anolis cybotes species complex). Evolution, 67: 3175–3190.
Wyles, J. S., & G. C. Gorman. 1980. The Classification of Anolis: Conflict between genetic and osteological interpretation as exemplified by Anolis cybotes. Journal of Herpetology, 14: 149–153.
Appendix 1. Specimens examined
Audantia aridius: Dominican Republic: Barahona: 2.9 miles NW La Ciénaga, 355 m: SMF 104162–63; Barahona: SMF 26145–48; Barahona, on ground of Hotel Riviera, 12 m: SMF 104910; 11.3 km S Barahona (measured from Hotel Caribe), 21 m: USNM 329093; 20.8 km S Cabral, 975 m: USNM 329090–92; Paraíso, ca. 6–7 km NW, 180 m: USNM 329089; Barahona, Hotel Costa Larimar, 10 m: SMF 97895; Los Lirios, 1110 m: SMF 97892–94; near Cortico, Laguna, 1340 m: SMF 97896; near Polo, 855 m: MNHNSD (GK–4838); Pedernales: 6.1 km S Los Tres Charcos, 5 m: SMF 104160–61.
Audantia armouri: Dominican Republic: Independencia: 16.7 km SE Puerto Escondido, 1690 m: USNM 575279; 23.1 km SE Puerto Escondido, 1690 m: SMF 104167; 23.9 km SE Puerto Escondido, 1690 m: SMF 104164–66, 104747–48, 104774, USNM 575275–78; Pedernales: Sierra de Bahoruco, El Gruce del Chucho, 2030 m: SMF 104751–56; Parque Nacional Sierra de Bahoruco, Canote – El Alcajé road, 1925 m: MNHNSD 23.3617, SMF 97820–23; 8.6 km NW Aceitillar (44.6 km N of Cabo Rojo), 1480 m: SMF 104731, 104749; Casetta Dos, ca. 22 km N Aceitillar by road, on ridge of Sierra de Bahoruco, 1750 m: SMF 104750, 104775, USNM 575280–81; 15 km SW El Aguacate, 2195 m: USNM 329119; 9.4 km S Aceitillar (26.6 km N Cabo Rojo), 710 m: SMF 104171. Haiti: Ouest: Morne Cardineau, 2060 m: SMF 104168–70, USNM 575282–83; Pic La Selle, 1927 m: SMF 104172–75, USNM 575284; Sud-Est: Gros Cheval, ca. 15 km W via logging roads (NE slope of Pic La Salle), 2020 m: USNM 286896; Gros Cheval, ca. 15 km W via logging roads (NE slope of Pic La Salle), 2020 m: USNM 329127–34.
Audantia australis: Dominican Republic: Pedernales: 18.2 km N. Pedernales at stream (Los Arroyos border road), 200 m: SMF 104274–75, USNM 575287; 9.4 km S Aceitillar (26.6 km N of Cabo Rojo), 710 m: SMF 104272–73, USNM 575285–86; Altagracia, 670 m: SMF 104276; Los Arroyos, 1265 m: SMF 97897; Mencía–Altagracia road, 1 km S Altagracia, 700 m: SMF 104277; road to Pelempito, 755 m: SMF 99003–08. Haiti: Grand’Anse: 8.0 km SSW Baradères, 420 m: SMF 104270, 104913; Bourdon, 9.2 km E Anse-d‘Hainault, 277 m: SMF 104914; Ouest: Berry, 1630 m: SMF 104278; Poye, 1285 m: SMF 104294, USNM 575291; Nippes: Morne Bois Pangnol, 1170 m: SMF 104291–92, USNM 575288; NW slope Morne Tête Boeuf, 1176 m: SMF 104287–88, USNM 575289–90; Sud: 11.6 km NW Les Anglais, on Morne Grand Bois, 1208 m: SMF 104285; Caye Michel, previously called Caye Paul (10.7 km WNW Les Platons Citadel), 1120 m: SMF 104269, USNM 575297; 2.4 km N Ducis, 50 m: SMF 104265–66, 104909, USNM 575292–94; 5.8 km S Ducis, 50 m: SMF 104267–68, USNM 575295–96; Île-à-Vache, 15 m: SMF 104284, USNM 575301–04; Île-à-Vache, Port Morgan Hotel, 16 m: SMF 104289–90, USNM 575305–07; Mme Verette, 1369 m: SMF 104293; near Cabalice, St. Jean, 107 m: SMF 104283; near Côteaux, 30 m: SMF 104281; ca. 12 km NE Port Salut, 189 m: SMF 104279–80, USNM 575298–300); Port Salut, Darbouze, 93 m: SMF 104282; Sud-Est: Jacmel (grounds of Hotel Jacmelian), 0 m: SMF 104271; Morne D’Enfer, 1433 m: SMF 104286, USNM 575308–09.
Audantia breslini: Haiti: Grand’Anse: 8.0 km SSW Baradères, 420 m: SMF 104194–95; Nord’Ouest: Bombardopolis, 490 m: SMF 104178–79, USNM 575310–13; Mole Saint-Nicolas at Rivière Côtes de Fer, 9.10 mi NE, 30 m: SMF 104177; 10.5 mi NE Mole Saint-Nicolas, 70 m: SMF 104176.
Audantia cybotes: Haiti: no further data: NHMD R3793, R3796; Grand’Anse: Jérémie: MCZ 14346–47; 1.8 km E Anse-d’Hainault, 130 m: SMF 104190–92, USNM 575314; 0.8 km E Dame-Marie, 40 m: SMF 104188–89, 104208; 4.8 km N Les Irois, 100 m: SMF 104193; 8.0 km SSW Baradères, 420 m: SMF 104194–95; 5.8 km S Pestel, 375 m: SMF 104187; 3.95 km WSW Annete, on Morne Desbarrières, 1623 m: SMF 104198; 1.5 km N Carcasse, 35 m: SMF 104197; Grande Cayemite (helipad-camp), 74 m: SMF 104201–03; Nippes: Morne Bois Pangnol, 1170 m: SMF 104204–05; Sud: 11.6 km NW Les Anglais, on Morne Grand Bois, 1208 m: SMF 104200; east slope Morne Grand Bois, 1100 m: SMF 104206–07, USNM 575320–21; near Côteaux: 7 m, SMF 104196; 5 km NW Duplantin, on Morne Lézard, Grande Colline, 1777 m: SMF 104199.
Audantia doris: Haiti: Artibonite: Gonave, Gran Source, 10 m: USNM 575322–23; Gonave, Nan Café spring area, 443 m: SMF 104210; Gonave: MCZ 13734–40; Gonave, near Richard on coast road, 7 m: SMF 104209.
Audantia higuey: Dominican Republic: Hato Mayor: 22 km WNW El Valle (16 km to Trepada Alta, ca. 6 km by trail to Montebonito), 76 m: USNM 329101–06; Sabana de La Mar, ca 10 km W (airline) in Los Haitises, 5 m: USNM 329107–10; La Altagracia: Hotel Catalonia Bávaro, 10 m: MNHNSD (GK–4670), SMF 97869; Loma El Peñon (García), ca. 5 km airline N Bejucal, 100–290 m: SMF 104257; Manatí Park Bávaro, 20 m: MNHNSD (GK-4694–95), SMF 97870– 72, 97874; San Cristóbal: vicinity of La Altagracia, W Santo Domingo, 40 m: SMF 99025.
Audantia hispaniolae: Dominican Republic: Azua: Playa Chiquita, 14.1 km NW Cruce de Ocoa on Baní–Azua road, then 4.8 km S, 9 m: SMF 104951, USNM 575324; Bahoruco: Apolinar Perdomo, 900 m: SMF 104226–27, USNM 575328–29; Loma Monte Bonito, 1800 m: SMF 104228–29, 104917–26, USNM 575325–27; Barahona:
Barahona; Loma del Curro, at crest of Sierra Martín García, 1061 m: SMF 104230–31, 104970–73, USNM 575330–32; Duarte: San Francisco de Macorís: SMF 22938; southern slopes of Loma Quita Espuela, 700–850 m: SMF 104221, 104959 60, USNM 575333–34; Batey Monati on east side of Río Payabo, 30 m: SMF 104915 16; El Seibo:
El Seibo, 110 m: SMF 97867–68; Loma Herradura (3 km NE of Pedro Sánchez, airline), 520–560 m: SMF 104220; 5.3 km SW Miches, 100 m: SMF104218; 1.7 km W Sabana de La Mar, 11 m: USNM 329094–95; Elías Piña: 0.5 km N. Calimete, 1560 m: SMF 104237; Elías Piña: 0.6 km NE Rosa de la Piedra, 1340 m: SMF 104236, USNM
575335; 1.1 mi. NE Rosa de la Piedra, 1220 m: SMF 104238, USNM 575336; Río Limpio (CREAR), 700 m: USNM 329100; 13 km N Cacique Enriquillo (27 km N Los Pinos), 1870 m: USNM 329096–98; 17 km N Cacique Enriquillo (31 km N of Los Pinos), 1450 m: USNM 329099; Espaillat: 23.2 km N Tenares, thence 4.5 km W (= 0.2 km E Jaiba), 350 m: SMF 104222; Moca: SMF 25925–26, 25928, 26341; Hato Mayor: Las Pajas: SMF 25826; Sabana de la Mar: SMF 26024, 26073; ca. 4 km S Sabana de la Mar, 36 m: SMF 104239, 104240, 104927–31, USNM 575337–39); Hermanas Mirabal: Tenares, 23.2 km N of, thence 4.5 km W (= 0.2 km E Jaiba): 350: SMF 104961; Independencia: ca. 7 km W Los Pinos by road, 365 m: SMF 104242; Cacique Enriquillo, 5.1 km N of, 1576 m: SMF 104232; west of Puerto Escondido, 490 m: SMF 104241; La Altagracia: Loma El Peñon (García), ca. 5 km airline
N Bejucal: 100–290 m: SMF 104219; La Vega: below Paso Bajito: SMF 25662, 25981– 87; between Moca and SMF 25903; Constanza: SMF 22935–36; Jarabacoa: SMF 25625, 25645–54, 25660–61, 25973–74; 10.5 km W Jayaco, 1000 m: USNM 329111; 11 km
W Jayaco on road to Constanza, 657 m: USNM 329118; 7 km W Jayaco on road to Constanza, 760 m: USNM 329113–17; Paso Bajito: SMF 34439; Reserva Científica Ébano Verde, Arroyazo, 1105 m: SMF 97898; Monte Cristi: Cayo Pablillo: SMF 25827–30; Monte Cristi: SMF 25841–42; Puerto Plata: 11 km from Puerto Plata: SMF 25929; 9 km from SMF 25927; 8.5 km NNE of Villa Elisa, 190 m: SMF 104964; above La Isabela, Río Isabela: SMF 25741; Balneario Colón, SMF 25871–80; Río Muñoz, 7 km from SMF 25898; 27 Cascadas, ca. 13 km airline WSW Puerto Plata, 165 m: MNHNSD 23.3630, 23.3632–33; Hotel Riu Bachata, ca. 6 km airline WNW Puerto Plata, 10 m: MNHNSD 23.3625; Loma Teleférico, ca. 1 km S Puerto Plata, 280–295 m: SMF 104758–60; near Yásica Arriba, 130 m: SMF 104761–64; 1.2–1.6 km SW Maimón, just west of mouth of the Río Maimón, 5 m: SMF 104223,
USNM 575340; Punta Burén, 6.4 km NW Estero Hondo, 6 m: SMF 104224–25, 104962–63; Samaná: Bahía Príncipe El Portillo, Península Samaná, 10 m: MNHNSD (GK–4705), (GK–5087), SMF 97875–77; El Limón, Península Samaná, 30 m: MNHNSD (GK–4709), (GK–4713), SMF 97878–85; Samaná: SMF 26048 54; Sánchez Ramírez: 8.6 km N of, thence, 8.6 km E of Cotuí (beyond Aguacate), 109 m: SMF 104978; 8.6 km NE of, thence 8.1 km E Cotuí (= El Aguacate), 35 m: SMF 104955 57; San José de Ocoa: Cruce de Ocoa, 14.8 N of, thence 7.8 km SE on dirt road; at Martínez, near La Palma, 675 m: SMF 104952–54; San Juan: 3.2 km NNE El Azul, 1000 m: SMF 104234–35, USNM 575341; San Pedro de Macorís: Consuelo: SMF 25716–24; Gran Bahia Principe La Romana, 10 km E San Pedro de Macorís, 10 m: MNHNSD (GK–4636), SMF 97863–66; San Pedro de Macorís: SMF 25697, 25709–10;
Santiago: Pass between Santiago and Puerto Plata: SMF 25868–69; Paso de Bao, 1300 m: USNM 575342; Guácara, 1169 m: SMF 104965–69; Monción: SMF 25759–65, 25785–94, 25798–801;Santo Domingo: Engombe at Río Haina: SMF 26125–27; Fortaleza at lower Río Haina: SMF 25525–27; Hotel Be Live Hamaca, Boca Chica, 10 m: SMF 97903; Hotel Don Juan, Boca Chica, 10 m: MNHNSD (GK–4739–40), (GK–4750), SMF 97886–91; Hotel Oasis Hamaca, Boca Chica, SMF 90421; km 17 Autopista Duarte, north of Santo Domingo, 55 m: SMF 97899–902; Mündung des Río Haina: SMF 25530–33; Río Haina: SMF 22937; Santa Ana, SMF 25547; Santo Domingo: SMF 22939–44; Santo Domingo Deutsch-Dominikanisches
Tropenforschungsinstitut: SMF 25511–12, 25518, 25610–11, 30009; Tres Ojos, SMF 25557. Haiti: Artibonite: 13.0 km N Ca Soleil (on road to Terre Neuve), 460 m: USNM 575344; 19.5 km N Ca Soleil, 620 m: SMF 104216–17, USNM 575343; Anse-a-Galets, Sunshine Hotel, 10 m: SMF 104248–50, USNM 575348–50; Gonave, Ravine Picme, near Platon Balais, 205 m: SMF 104245–47, 104977, USNM 575345–46; Morne Basile, 1424 m: SMF 104253–55, USNM 575352–54; Morne Boeuf, 1760 m: SMF 104256, USNM 575351; Saint-Marc: SMF 26239–40; Centre: 16 km N Croix-des-Bouquets, 510 m: SMF 104215; Bonga, 1263 m: SMF 104244; ca.
4 km NW Ca Pierre by road, 1200 m: SMF 104251, 104975, USNM 575363; Chinchiron, 1154 m: SMF 104252, 104976; Kenskoff: SMF 26245–47; Nord: Cap-Haïtien: SMF 10867– 913, 26249–50; Nord’Ouest: 1.1 miles S Douceur (8 mi E Port-de-Paix), 20 m: SMF 104233, USNM 575359; Ouest: Furcy (at The Lodge), 1575 m: SMF 104243, 104932–45, 104974, USNM 575360–62; Port-au-Prince: SMF 10919–30; Bernard (east of Robin and Furcy), 1300 m: SMF 104213–14, 104772, 104947, USNM 575357–58; 12.0 km ENE Petionville, 165 m: SMF 104950; 4.2 km E Carisse, by road, 210–260 m: SMF 104211, 104949, USNM 575356; 18.1 km E Thomazeau, 800 m: SMF 104212, 104948, USNM 575355.
Audantia longitibialis: Dominican Republic: Pedernales: Bucan Detwi, 10 m: USNM 575370; 25.2 SE Pedernales, 180 m: SMF 104979; Isla Beata, ca. 2 km SE Punta Beata, 10 m: SMF 104263–64, USNM 575366–69; ca. 5 km SW Los Tres Charcos (ca. 0.5 km NW Fondo de Paraíso), 85 m: SMF 104258–59, USNM 575371.
Audantia ravifaux: Dominican Republic: La Altagracia: Hotel Catalonia Bávaro, 10 m: SMF 97869; Manatí Park Bávaro, 20 m: SMF 97873; La Romana: Isla Saona: MCZ R-37468–69, 187412–17; Isla Saona, environs of Mano Juan: MCZ R-156221.
Audantia saxatilis: Dominican Republic: Azua: Manantiales, 485 m: SMF 104303; Barahona: south of Fondo Negro, region of lower Río Yaque: SMF 25032; Independencia: N of La Descubierta, 134 m: SMF 104300. Haiti: Nord’Ouest: Mole Saint-Nicolas at Rivière Côtes de Fer, 9.10 mi NE, 30 m: USNM 575374; 1.5 mi WSW Mole Saint-Nicolas (along coast), 35 m: SMF 104302, USNM 575373; 6.3 mi W Port-de-Paix, 32 m: SMF 104301, USNM 575372.
Audantia shrevei: Dominican Republic: La Vega: 13 km NW La Horma, 1770 m: USNM 329112; Santiago: Valle de Bao, 1800 m: SMF 104295–97, USNM 575375–76.
Audantia strahmi: Dominican Republic: Independencia: west of Puerto Escondido, 490 m: SMF 104299, USNM 575377–78; Pedernales: Hoyo de Pelempito (H. de Aceitillar on topo map) east edge, 355 m: SMF 104298, 104773; road to Pelempito, 315 m: SMF 97997–99, MNHNSD 23.3615–16.
Appendix 2 . GenBank accession numbers for all sequences included in the study. The specimen ID is shown in the tree (Fig. 1) and is the laboratory sample number (SBH), previously published Genbank number (AY), or museum number: KU, Museum of Natural History, Kansas University; MVZ, Museum of Vertebrate Zoology, University of California, Berkeley; USNM, U. S. National Museum, Smithsonian; SMF, Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt. Corresponding Museum IDs are given. N/A = not applicable (no sequence).
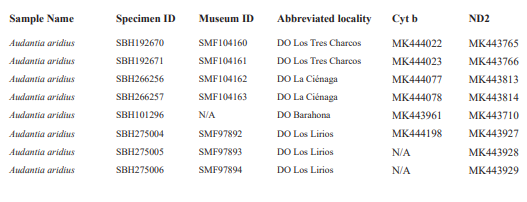
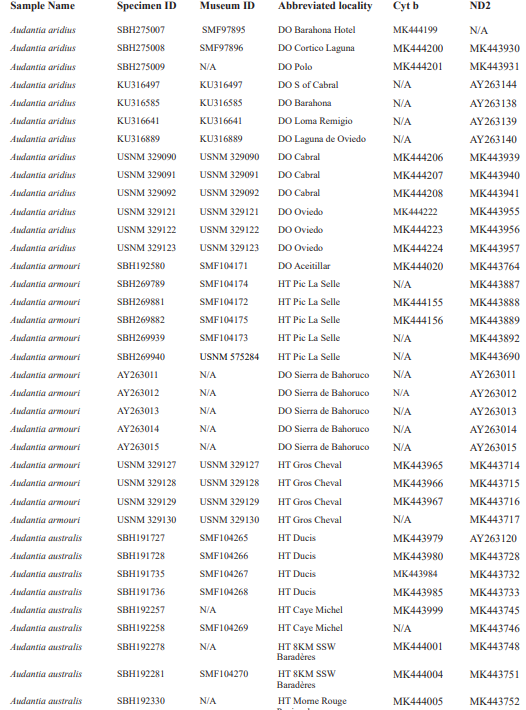
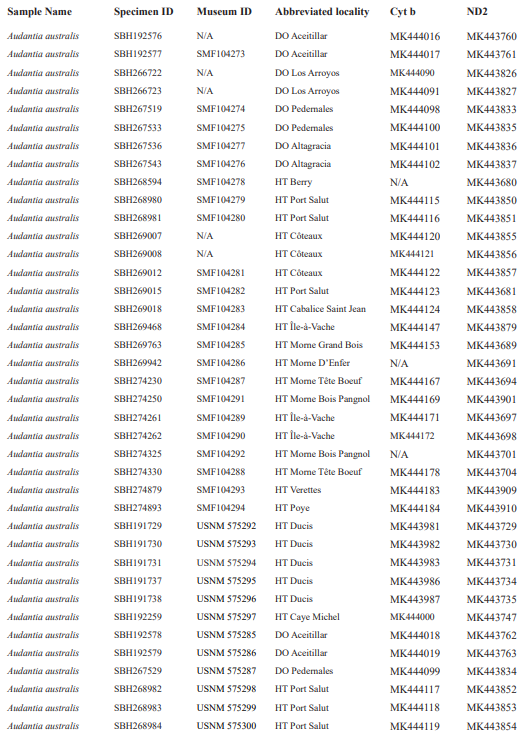
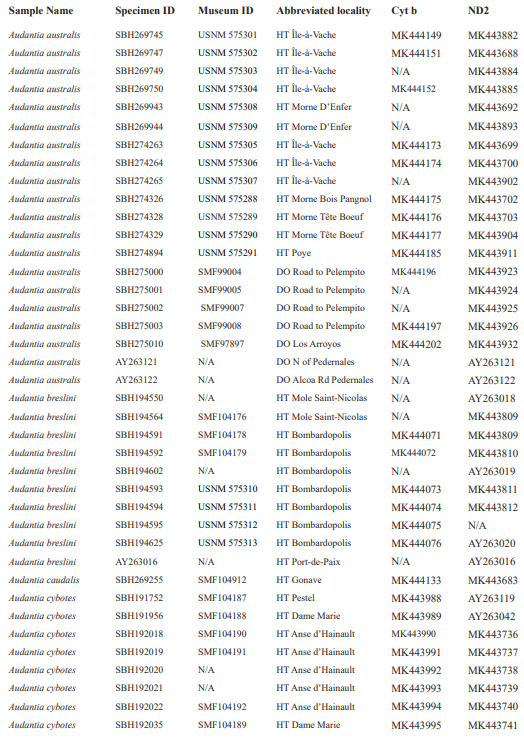

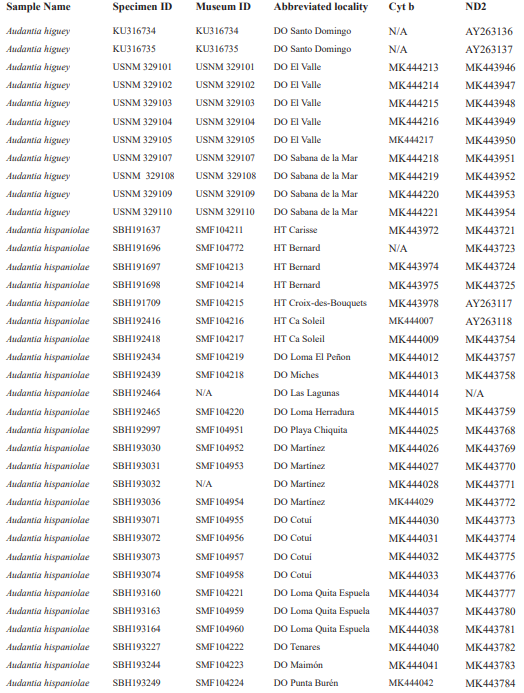
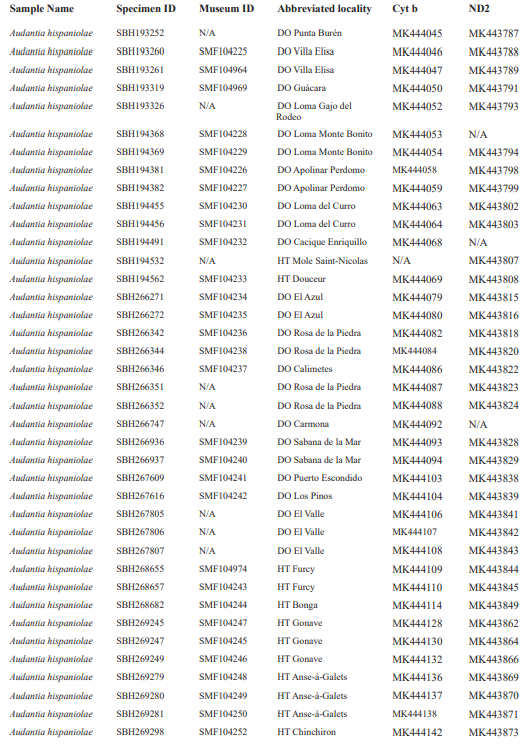
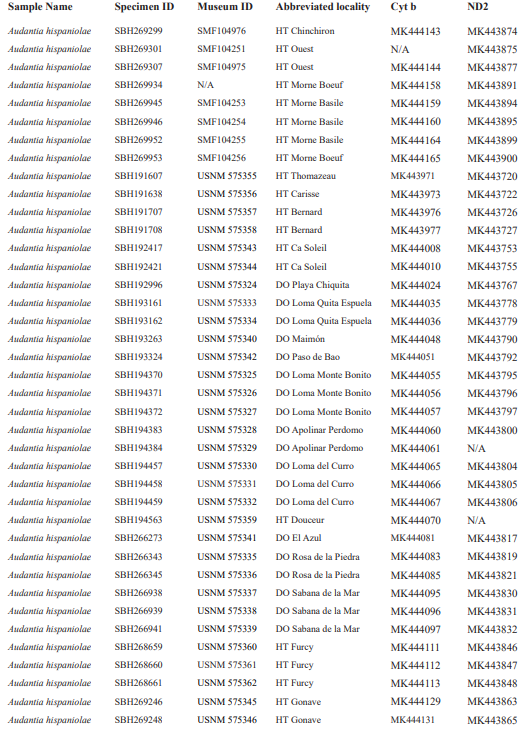
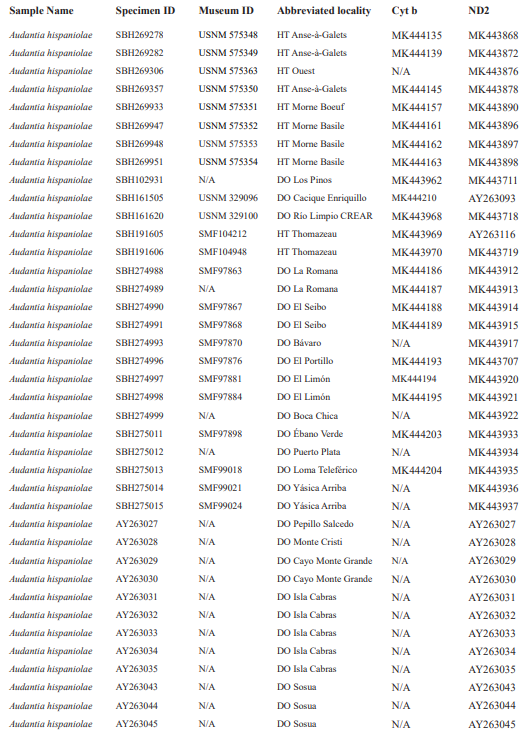
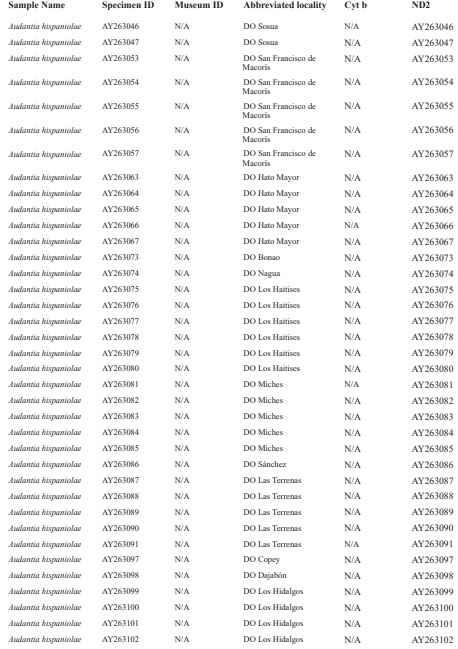
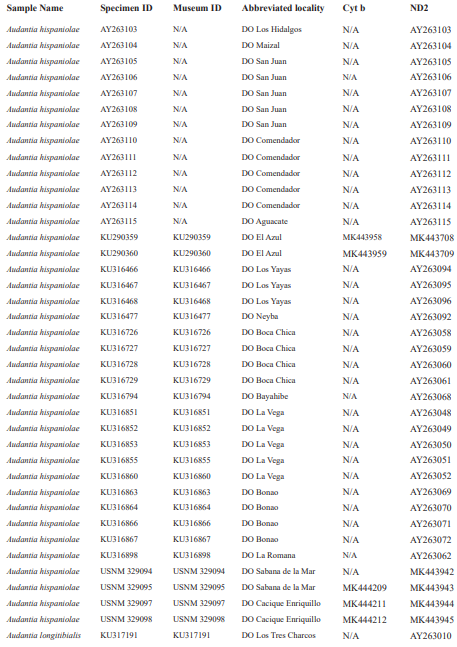
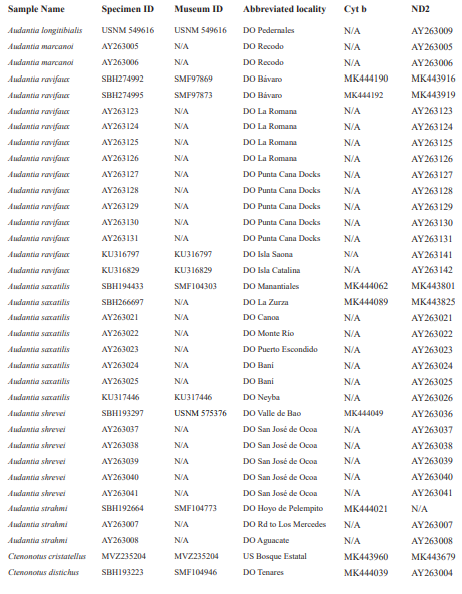
Cómo citar:doi: Köhler, G., Zimmer, C., McGrath, K., & Hedges, S. B. (2019). Una revisión del género Audantia de la Hispaniola con descripción de cuatro especies nuevas (Reptilia: Squamata: Dactyloidae). Novitates Caribaea, (14), 1–104. https://doi.org/10.33800/nc.v0i14.201