![]() Número 22,
julio, 2023: 13–24
Número 22,
julio, 2023: 13–24
ISSN versión impresa: 2071–9841 ISSN versión en línea: 2079–0139 https://doi.org/10.33800/nc.vi22.336
POPULATION ESTIMATES OF BAT ASSEMBLAGES FROM HOT CAVES IN PUERTO RICO
Estimados poblacionales de asociaciones de murciélagos en cuevas calientes de Puerto Rico
Armando Rodríguez-Durán1a*, Natalie A. Nieves1b, Yadiamaris Avilés Ruiz1c, Yaniré Martínez2 and Kamile Andújar-Morales1d
![]() 1 Universidad Interamericana, Recinto
de Bayamón, Puerto Rico; a https://orcid.org/0000-0002-4688-0758; bhttps://orcid.org/0000-0002-6101-8306, natalieann3.14@gmail.com;
c https://orcid.org/0000-0001-9009-2495, yadiamaris.aviles@upr.edu;
d https://orcid.org/0000-0002-3615-7603, kamile.andujar@gmail.com. 2 Programa de Conservación
de Murciélagos de Puerto Rico, Bayamón, Puerto Rico; https://orcid.org/0000-0002-9435-6010, yanirem@gmail.com. *Corresponding author:
arodriguez@bayamon.inter.edu.
1 Universidad Interamericana, Recinto
de Bayamón, Puerto Rico; a https://orcid.org/0000-0002-4688-0758; bhttps://orcid.org/0000-0002-6101-8306, natalieann3.14@gmail.com;
c https://orcid.org/0000-0001-9009-2495, yadiamaris.aviles@upr.edu;
d https://orcid.org/0000-0002-3615-7603, kamile.andujar@gmail.com. 2 Programa de Conservación
de Murciélagos de Puerto Rico, Bayamón, Puerto Rico; https://orcid.org/0000-0002-9435-6010, yanirem@gmail.com. *Corresponding author:
arodriguez@bayamon.inter.edu.
[Received: February 20, 2023. Accepted: April 19, 2023]
ABSTRACT
Knowledge about the number of bats roosting in caves is critical for their long-term conservation. These roosts may function as shelter from weather or predators, information and social centers, or as nurseries. We provide estimates of bat population from 12 hot caves in Puerto Rico, based on infrared reflectance technology. Populations ranged from a few thousands of individuals to several hundred thousand. At nine caves, estimates were performed twice at different periods during the reproductive cycle. Some caves showed notable variations in the size of the population. A database of caves used by bats throughout the island is also provided, as well as information on the species that have been identified at each cave.
Keywords: West Indies, Antilles, population size, seasonality, reproduction, conservation.
RESUMEN
El conocimiento sobre la cantidad de murciélagos que se albergan en cuevas es fundamental para desarrollar medidas de conservación de largo plazo. Estos albergues ofrecen protección contra las inclemencias del tiempo y depredadores, y pueden servir como centros de información, de actividad social, así como guarderías. En este trabajo, utilizamos tecnología de reflectancia infrarroja para estimar el tamaño poblacional en 12 cuevas calientes en Puerto Rico. Las poblaciones en las cuevas variaron desde unos pocos miles de individuos, hasta cientos de miles. En nueve de estas cuevas, los estimados se llevaron a cabo en dos ocasiones, tomando en cuenta la temporada reproductiva, algunas de ellas mostrando variaciones notables en el tamaño de sus poblaciones. Ofrecemos también una base de datos de cuevas usadas por murciélagos a través de la isla, así como información sobre las especies que habitan en cada una de ellas.
Palabras clave: Indias Occidentales, Antillas, tamaño poblacional, estacionalidad, reproducción, conservación
INTRODUCTION
The importance of roosts in the life histories of bats has been extensively reviewed (Kunz, 1982; Kunz & Lumsen, 2003). Roosts may function as shelter from weather or predators, information or social centers, or as nurseries. Forty-five of the 61 extant species of bats on the West Indies are endemic to the archipelago, with a large percentage of species roosting in caves as compared to mainland assemblages (Kurta & Rodríguez-Durán, 2023; Rodríguez-Durán & Kunz, 2001). Of the bat fauna in Puerto Rico, 80% are cave-dwelling species. Many of these cave-dwelling species roost in hot-caves, where they form non-random assemblages (i.e., only specific combinations of species are found in a cave [Rodríguez-Durán, 1998]) and may function as physical ecosystem engineers, changing the temperature and gaseous composition inside the cave (Ladle et al., 2012; Rodríguez-Durán, 2009), in addition to importing nutrients.
The abundance of caves is particularly high in karstic areas throughout the world, where they may serve as important species reservoirs (Furey & Racey, 2016). Different types of caves develop in the karst regions of the Greater Antilles, including fluvio-karst caves, formed by rivers or by rainfall that percolates through the limestone (Lugo et al., 2001). Flank margin caves form in the distal margin of a freshwater lens, where freshwater and seawater mix and produce dissolution-aggressive water (Gamble et al., 2000). Large or medium-sized caves, typically fluvio-karst caves, allow for the formation of hot caves. These large caves can have several kilometers of passages and large populations of bats, and may contain chambers large enough to accommodate a small cathedral inside. Medium sized caves may be several hundred meters long with turns and chambers over 15 m high and may also contain hot chambers. But different types of hot caves exist in various parts of the globe. Some may be heated geothermally (Bell et al., 1986), others due to convection of hot air entering the cave through its opening, or entrapment of heat generated by bats (Ladle et al., 2012; Rodríguez-Durán, 2009; 2020). All hot caves known from the West Indies pertain to this latter category. Bats of the family Mormoopidae, Phyllostomidae, and Natalidae represent the main taxa associated with hot caves in the Neotropics (Gannon et al., 2005; Rodríguez-Durán, 1998; 2005; Silva-Taboada, 1979). In the West Indies, endemic mormoopids and phyllostomids, such as those in the genera Pteronotus, Mormoops, Monophyllus and Erophylla, show reduced metabolic rates and renal adaptations to life in these hot-caves (Rivera-Marchand & Rodríguez-Durán, 2001; Rodríguez-Durán, 1995).
Although caves are not uniformly distributed throughout the islands, they are ubiquitous in karst regions. However, many caves are not used by bats as day roosts, while others contain multi-species assemblages in the tens or hundreds of thousands of individuals (RodríguezDurán, 1998). The ecological services provided by such large populations, such as flux of energy and nutrients in ecosystems, pollination, seed dispersal, and control of insects, have been documented both in Puerto Rico and elsewhere (Boyles et al., 2011; Kunz et al., 2011; Rodríguez-Durán & Lewis, 1987). By aggregating in such a way, bats are likely to increase their travel time to feeding grounds (Rodríguez-Durán, 2009; Silva-Taboada, 1979), because the average time spent traveling to and from the cave is proportional to the average trip length. This is time and energy lost, relative to the time that would have been spent if foraging had occurred in the area immediately around the cave.
One advantage of a multi-species assemblage is the modification of the cave’s microclimate, provided that the characteristics of the site allow such modification. Various ecological processes may promote the formation of large multispecies assemblages in caves. Differences in peak exit times of emergence, associated with different temporal patterns of foraging (e.g., RodríguezDurán & Lewis, 1987), may allow larger numbers of heat producing bodies to be present than would be possible in either a monospecific colony or a random assemblage of species, in which peak exit times by different species might coincide. Such large groups may provide active benefits by promoting the development and maintenance of a thermoneutral environment inside the cave (Rodríguez-Durán, 1995).
Strong hurricanes may have catastrophic effects on bat populations, although typically most islands receive direct devastating impacts from hurricanes of Category III or higher only occasionally. Under these circumstances, caves, in addition to providing an energetically advantageous roost environment, are likely to provide protection against the milder but more frequent climatic disturbances and appear to be central to the formation of bat assemblages in the West Indies (Rodríguez-Durán, 2009; Rodríguez-Durán & Kunz, 2001). Thus, hot caves with large populations of bats likely represent irreplaceable critical refugia that needs to be understood and protected (Padilla-Rodríguez, 2021). Estimating the number of bats roosting in caves is critical for their long-term conservation (Kunz et al., 2009). However, before this study, only two caves had been censused on the island of Puerto Rico.
OBJECTIVES
- The main objective of this project was to estimate the population size of large bat colonies roosting in caves in Puerto Rico. The census and monitoring presented here will serve as baseline for the development of management and conservation practices.
MATERIALS AND METHODS
In this survey, the population of bats at nine hot caves was estimated and monitored (Hayes et al., 2009) by censusing each population twice. Three additional hot caves were censused once. For the nine caves that were censused twice, each estimate was performed on a different season, as related to the reproductive cycle of bats. Estimates at the additional three caves occurred after parturition season. All the work was performed within the karst region on the main island of Puerto Rico (Table I). The nine caves censused twice were: Adrobel (Camuy), Cucaracha and Madama (Aguadilla), Culebrones (Arecibo), Jiménez (Manatí), Humo (Vega Alta), Pérez (Isabela), Volcán (Florida) and Cueva 42 (Utuado). The three additional caves were: Matos (Arecibo), Murciélagos (Cayey), and Efraín López (Isabela).
The morphological complexity of hot caves combined the large number of bats inhabiting them, makes impractical the direct counting inside the cave during the day. Thus, the number of bats were estimated at each selected hot cave by modifying the methods described by Rodríguez-Durán & Lewis (1987), through continuous recording using infrared light rather than photographing exit activity with visible light flashes. A tripod-mounted, reflectance, Ancter Full Spectrum HD infrared night vision camera, with supplemental infrared sources, was positioned so that emerging bats flew perpendicular to the field of view (Kunz et al., 2009). The number of bats counted was extrapolated to the full volume of the cave opening, following RodríguezDurán & Lewis (1987). Incorrect determination of the exit area used by bats represents the main potential error to these estimates. Additionally, flash photographs of exit activity were obtained on separate dates for the first nine caves, during March 2022, to estimate the proportion of different species occupying them. Photos were taken at regular intervals and bats in each photo identified to species. Unidentifiable individuals were not considered for calculations.
Each census began at the onset of bat activity, and for the duration of the evening exodus (Rodríguez-Durán, 1996; Rodríguez-Durán & Lewis, 1987) following standard protocol as described by Kunz et al. (2009). Based on observation of exit behavior, it was determined that the evening exodus had ended when four consecutive, one-second sampling units, showed no bats. Flight speed was not used to estimate population size, but rather the average number of bats per second was combined with the total duration of the evening exodus.
Multiple caves were considered during initial evaluation to determine which ones were suitable for estimating population size. Information on all caves, whether it was finally used for the estimates, is included in Table I. Caves used to conduct the estimations showed: 1) morphological parameters of the openings that allowed filming all bats exiting; 2) at least one chamber with a temperature of over 25 °C, which defines a hot cave; and 3) at least one of the species that characterizes a hot cave, as described in the introduction, used the cave as day roost. Geographic locations were taken using a Garmin ETREX 22X GPS, distances measured with a Bosch Professional GLM 30, and temperatures with a NDIR CO2 Meter (Model 7755) and a Glass organic filled thermometer.
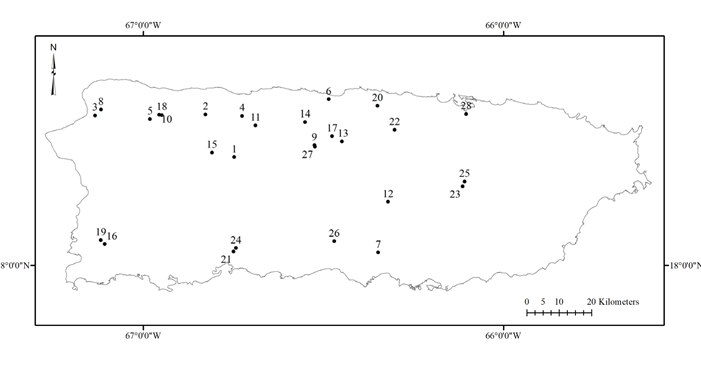
Figure 1. Distribution of caves considered for the study. Numbers of caves correspond to those in Table I.
Table I. List of caves and known species of bats inhabiting them at the time of the study. The first 12 caves were used for the estimates of population size. Species highlighted in bold represent the most abundant species in these 12 caves at the time of the survey. Species identified included: Aj, Artibeus jamaicensis; Bc, Brachyphylla cavernarum; Eb, Erophylla bombifrons; Ef, Eptesicus fuscus; Mb, Mormoops blainvillei; Mr, Monophyllus redmani; Nl, Noctilio leporinus; Pp, Pteronotus portoricensis; Pq, Pteronotus quadridens.
|
Name |
Latitude |
Longitude |
Municipality |
Species identified |
|
1 Cueva 42 |
N 18°18’01.9332” |
W 66°44’51.1188” |
Utuado |
Pp, Bc, Aj, Mb |
|
2 Adrover |
N 18°25’07.0140” |
W 66°49’38.0856” |
Camuy |
Pq, Mr, Mb, Pp |
|
3 Cucaracha |
N 18°24’57.5424” |
W 67°08’02.1984” |
Aguadilla |
Pq, Mb, Mr |
|
4 Culebrones |
N 18°24’52.5384” |
W 66°43’31.7676” |
Arecibo |
Pq, Mb, Bc, Mr, Eb, Pp |
|
5 Pérez |
N 18°24’20.8476” |
W 66°58’52.8996” |
Isabela |
Bc., Pq., Aj. Eb. Mr., Pp. |
|
6 Jiménez |
N 18°27’36.1980” |
W 66°29’06.3744” |
Manatí |
Aj, Pq , Mr, Eb, Ef |
|
7 Humo |
N 18˚22’07.2000” |
W 66˚20’52.5000” |
Vega Alta |
Mb, Aj, Eb, Pp, Pq, Mr |
|
8 Madama |
N 18˚25’53.1000” |
W 67˚07’03.1000” |
Aguadilla |
Eb, Mr, Pp, Pq, Mb, Aj |
|
9 Volcán |
N 18˚20’01.4000” |
W 66˚31’29.8000” |
Florida |
Mr, Pq, Eb, Pp, Mb |
|
10 Efraín López |
N 18°25’06.0816” |
W 66°57’20.8116” |
Isabela |
Aj, Pq, Eb |
|
11 Matos |
N 18°23’18.1212” |
W 66°41’19.2624” |
Arecibo |
Aj, Bc, Eb, Nl |
|
12 Murciélago |
N 18°10’34.5000” |
W 66°19’13.5000” |
Cayey |
Bc |
|
13 Buruquena |
N 18°20’38.5260” |
W 66°26’55.8168” |
Morovis |
Aj, Pq |
|
14 Monchocolo |
N 18°23’51.5832” |
W 66°33’03.2760” |
Florida |
Mb, Aj, Ef, Pq |
|
15 Dugón |
N 18°18’46.8396” |
W 66°48’33.9660” |
Utuado |
Bc, Aj, Mr, Mb |
|
16 Malano |
N 18°03’31.1544” |
W 67°06’26.1612” |
Cabo Rojo |
Ef, Aj, Mb, Pq |
|
17 M. d Plátano |
N 18°21’31.2264” |
W 66°28’32.9160” |
Ciales |
Aj |
|
18 Ortega |
N 18°25’01.0524” |
W 66°56’55.9356” |
Isabela |
Aj, Bc, Pq, Mb |
|
19 Tuna |
N 18°04’11.6760” |
W 67°07’04.0224” |
Cabo Rojo |
Aj, Bc, Mb, Eb, Mr, Pq |
|
20 Ortiga |
N 18°26’31.2504” |
W 66°20’59.8812” |
Vega Baja |
Pq, Aj |
|
21 Convento |
N 18°02’17.0700” |
W 66°44’57.2600” |
Guayanilla |
Bc, Mr, Ef, Pq, Pp |
|
22 Bonita |
N 18°22’34.0000” |
W 66°18’08.0000” |
Toa Alta |
Bc, Eb, Mr, Pq, Pp |
|
23 Grillos |
N 18°13’07.9300” |
W 66°06’49.6600” |
Agua Buenas |
Eb, Mr, Pp, Pq |
|
24 Mapancha |
N 18°02’51.0000” |
W 66°44’33.0000” |
Peñuelas |
Aj, Pp |
|
25 Murciélago |
N 18°13’56.9600” |
W 66°06’28.5600” |
Guánica |
Bc, Mr, Aj, Mb, Pq, Pp |
|
26 Naranjo |
N 18°03’59.5000” |
W 66°28’10.9700” |
Juana Díaz |
Eb, Mr, Mb, Pp, Pq |
|
27 Vientos |
N 18°19’44.8000” |
W 66°31’25.2000” |
Florida |
Pp, Pq, Eb, Mr |
28 Canejas N 18˚25’11.3000” W 66˚06’14.0000” Guaynabo Aj, Mr
RESULTS
Based on the evaluation of potential sites, we provide a database of caves used by bats throughout the island, as well as information on the species identified at each cave (Table I; Fig. 1). Some caves include all three sections of the temperature gradient, thus explaining the presence of species not typical of hot caves. We did not consider some hot caves with apparent large populations, especially along the southern coast, because the morphology of their exits did not allow for good estimates. Temperatures reported indicates minimal temperature measured in hot chambers. Higher and lower temperatures than those reported are present in most caves. These variation in temperature may occur due to changes in number of bats, presence of microstructures, variations in flow of water, and elevation above ground level. Of all caves censused twice, four showed substantial seasonal reduction in the size of their populations, ranging from 48% to almost 100% (Table II): Cucaracha (52%), Culebrones (48%), Jiménez (80%) and Cueva 42 (≈ 100%). Flash photographs of exit activity revealed, in some instances, species not previously reported for some caves, or failed to reveal species previously reported.

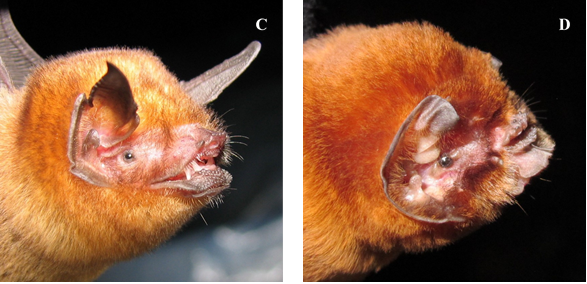
Figure 2. The most common bats defining hot caves include the phyllostomids E. bombifrons (A) and M. redmani (B), and the mormoopids P. quadridens (C), and Mormoops blainvillei (D). Photos: J. Angel Soto-Centeno.
Table II. Estimated population size, temperature, and date of each census. Numbers in parenthesis indicate the relative abundance of each species as a percentage of the population in the cave, where (<1) indicates that the species has been reported at the cave but was not detected in photos. See Table I for abbreviation of species names.
|
Cave |
Temp °C |
Estimate 1 |
Date |
Estimate 2 |
Date |
Percentage contribution of each species March 2022 |
|
Jiménez |
26 |
322 057 |
Sept/13/20 |
66 433 |
Apr/20/21 |
Aj (7), Pq (78), Mr (13), Eb (2), Ef (<1) |
|
Cucaracha |
26-35 |
690 606 |
Sept/19/20 |
358 910 |
Mar/13/21 |
Pq (50), Mb (45), Mr (5) |
|
Madama |
26 |
37 584 |
Sept/20/20 |
21 929 |
Mar/14/21 |
Eb (<1), Mr (44), Pp (12), Pq (14), Mb (23), Aj (7) |
|
Culebrones |
26-32 |
158 300 |
Oct/15/20 |
76 028 |
Mar/5/21 |
Pq (53), Mb (32), Bc (3), Mr (3), Eb (1), Pp (8) |
|
Humo |
30 |
12 355 |
Oct/22/20 |
15 422 |
Mar/7/21 |
Mb (16), Aj (<1), Eb (10), Pp (13), Pq (13), Mr (48) |
|
Pérez |
24-28 |
80 166 |
Oct/23/20 |
72 180 |
Mar/12/21 |
Bc (20), Pq (30), Aj (8) Eb (9) Mr (4), Pp (29) |
|
Volcán |
26-28 |
533 576 |
Oct/25/20 |
548 280 |
Apr/10/21 |
Mr (1), Pq (78), Eb (1), Pp (14), Mb (6) |
|
Cueva 42 |
26 |
51 528 |
Nov/8/20 |
27 |
Apr/11/21 |
Pp (14), Bc (15), Aj (20), Mb (51) |
|
Adrover |
26-33 |
164 502 |
Nov/14/20 |
179 846 |
Mar/6/21 |
Pq (91), Mr (1), Mb (5), Pp (3) |
|
Murciélagos |
24-26 |
N/A |
N/A |
7156 |
Sept/11/21 |
N/A |
|
Matos |
26 |
N/A |
N/A |
48 876 |
Oct/5/21 |
N/A |
|
Efraín López |
26 |
N/A |
N/A |
5447 |
Oct/12/21 |
N/A |
DISCUSSION
Despite the use of modern techniques, such as doppler radar or thermal infrared videos, efforts to obtain exact measurements of population sizes of cave dwelling bats have proven difficult to achieve (Furey & Racey, 2016). Caves for this study were selected because they contained at least one hot chamber (Table II). However, the final decision on whether using a cave to estimate the population of bats was a function not only of thermal characteristics and perceived size of the population, but also whether the morphology of the cave opening allowed for a reasonably accurate estimate. Factors such as number, size, and shape of the openings, influence the potential accuracy of estimates. Also, multispecies colonies of bats show continuous and overlapping activity throughout the night. All these factors can potentially induce error in the estimates. Thus, in estimating population size, it is important to make an appropriate selection of sites and educated decisions regarding the duration of the evening exodus of bats. Also, the fact that a species previously reported for a cave (Gannon et al., 2005) was not detected by the photographs, does not necessarily mean that it is no longer present at that cave. Species that roost in small numbers, such as Eptesicus fuscus, are likely to be missed by the method used in this study. Likewise, species observed in photographs in fair numbers but not detected in the cave during the day, could be using the site as a night roost. Flash photographs for the purpose of identification of species were not taken at the same time as recordings to estimate population size. Thus, due to the seasonal variations observed in the number of bats in caves, in trying to obtain an absolute number of individuals per species, the percentage contribution of each species in Table II is likely to be more accurate when applied to the second estimate of population size in that same table (March – April). An exception to this suggestion would be Cueva 42, where the size of the population during the second census was exceedingly small.
Seasonal and annual variations in the number of bats, and composition of assemblages in caves, was expected before the census, based on historical observations (AR-D pers obs). The reason for these variations is not known with certainty, although it may be mainly related to reproductive activity. There is evidence of sexual segregation in caves during the breeding season, where one of the sexes moves to different chambers within the cave, or to a completely different cave (Silva-Taboada, 1979; Rodríguez-Durán & Lewis, 1987). One of the caves censused twice for this study (Cueva 42) showed a population of over 50 000 individuals on the first visit, but just under 30 bats the second time. Thus, supporting the interpretation that variation observed at other caves was not the result of errors in the estimates, but of real shifts in the number of bats occupying those caves throughout the year. The reduced number of individuals at Cucaracha Cave during the March census (Table II) could be the result of sexual segregation prior to parturition (Rodríguez-Durán & Lewis, 1987). The large number of individuals in September could result from the reunition of sexes during this season, combined with the contribution of recently weaned bats. The other five caves where estimates were carried out twice (Madama, Humo, Pérez, Volcán, and Adrover), showed small variations in number of bats. Such small variations could indicate that sexual segregation occurs in separate rooms within the same cave (Silva-Taboada, 1979). Thus, maintaining the total population size almost unaltered throughout the year.
In Puerto Rico, the population of only two caves had been estimated before. Cucaracha Cave had an estimated population of about 700 000 individuals in 1983 (Rodríguez-Durán & Lewis, 1987), whereas at Culebrones Cave the population in 1996 was estimated at about 300 000 individuals (Rodríguez-Durán, 1996). The results from Cucaracha Cave suggest that, overall, it has maintained a stable population over the past 40 years and remains as one of the largest populations known on the island. The variation among estimates at Cucaracha in this study can be attributed to seasonal variations. To some extent this stability with respect to the 1983 estimate is unexpected, given the known impact of major hurricanes over the past 40 years (Jones et al., 2001; Rodríguez-Durán, 2009). However, flash photographs suggest that the proportion of the different species has changed relative to those estimated in 1983. On the other hand, Culebrones cave shows a smaller population when compared to the 1996 census (Rodríguez-Durán, 1996). An expected result, given the large decline in what used to be the most abundant species at this cave, Erophylla bombifrons, documented after Hurricane Georges (Jones et al., 2001; Rodríguez-Durán, 2009). This hurricane caused not only high mortality rates, but also shifts in species composition within the cave. Although the population of E. bombifrons slowly started to recover since Hurricane Georges, it was reduced again by Hurricane María (AR-D lab, unpubl. data). These differences between Cucaracha and Culebrones caves could be related to the differences in their species composition, and how those species are affected by hurricanes. As with Culebrones cave, the most abundant species at Cucaracha cave was also a phyllostomid bat (M. redmani). However, at Culebrones Cave M. redmani recovered more quickly after Hurricane Georges than E. bombifrons (Rodríguez-Durán, 2009). Even though the population size of M. redmani appears to be much smaller at Cucaracha in 2022, compared to 1983, it is the most abundant species at nearby Madama cave, complicating the interpretation of these results. Regular movement of bats among these two caves have been speculated since the initial studies in 1983.
Variations in the size of local populations support previous hypotheses regarding the apparently fluid nature of cave use by bats, and stresses the importance of protecting caves in general, irrespective of bat population size. Caves that appear uninhabited or holding small populations at one time, could become important refugia at another season. The dynamic nature of bat assemblages in caves was proposed by Rodríguez-Durán (2009) in relation to how different species react differently to tropical storms. Populations of some cave bats may be kept in check, and others may be allowed to persist, because of periodic storms that impede one species from displacing the other. For instance, both E. bombifrons and Pteronotus portoricensis occupy the Tepidarium of caves (Kurta & Rodríguez-Durán, 2023; RodríguezDurán & Christenson, 2012). At Culebrones Cave, Hurricane Georges, in 1998, reduced the population of the phytophagous E. bombifrons, which may have facilitated occupation of the site by the insectivorous P. portoricensis. In the 20 years after Georges, P. portoricensis, the new species, continued to thrive, but the population of E. bombifrons, once the most abundant species in the cave, had not fully recovered to pre-Georges levels before it was downsized again by Hurricane María in 2017.
Bats in hot caves are known to disperse over areas of more than 10 km around their roosts (Rodríguez-Durán & Lewis, 1987; Silva-Taboada, 1979), and connectivity of these habitats is important for bat conservation in Puerto Rico (Calderón-Acevedo et al., 2021). The dynamic nature of populations of bats in caves suggests that alteration or loss of caves with apparent low value as bat roosts, could lead to disruption of this landscape connectivity. In addition, large populations in hot caves cannot be sustained without adequate foraging areas. Deforestation in the areas surrounding these caves will require longer commutes to suitable habitats, or smaller populations, thus reducing the viability of the colonies.
CONCLUSION
The ecological importance of hot caves is well documented (Ladle et al., 2012), not only for bat conservation, but for a myriad of other organisms. Probably the major lesson learned from this survey is that the interaction of bat assemblages and roosts is even more complex than previously thought. Although the impact of hurricanes on phytophagous bats is well documented (Jones et al., 2001), care must be applied when interpreting sporadic rather than continuous censuses (e.g., Adams, 2001). The results from this study suggest that careful detailed examination of caves needs to be made before determining their importance as a bat roost, even where anecdotal observations suggest little or no use by bats. A complete evaluation of the importance of a cave for bats requires a determination of its possible use as a seasonal day roost, as well as a night roost during foraging bouts. These examinations will require monthly observations, to determine whether the cave is occupied part of the year. Bats roosting in hot caves disperse over long distances to forage during the night (Rodríguez-Durán, 2009; SilvaTaboada, 1979), and the availability of night roosts during those foraging bouts is essential for their survival, providing for a place where the bat rests between foraging bouts without having to fly all the way back to the day roost. Monthly censusing of caves that have shown large population variations in this survey would be desirable, to better understand the dynamics of bat assemblages that inhabit them.
Finally, hot caves are not the majority of caves, and represent an irreplaceable habitat for at least five species of bats in Puerto Rico (Padilla-Rodríguez, 2021; Rodríguez-Durán, 1998). Caves hosting these species (E. bombifrons, M. redmani, P. portoricensis, P. quadridens, and Mormoops blainvillei), together with surrounding foraging grounds and corridors, should be protected and considered of greatest conservation need for their survival.
ACKNOWLEDGEMENTS
This work was possible thanks to the support provided by the Departamento de Recursos Naturales y Ambientales de Puerto Rico and the United Sates Fish and Wildlife Service. The Universidad Interamericana de Puerto Rico, Bayamón Campus (UIPR-BC), provided additional release time. The Programa de Conservación de Murciélagos de Puerto Rico and students from UIPR-BC assisted with field work and some analyses. Finally, thanks to the two anonymous reviewers and the editor, Gabriel de los Santos, who offered inputs that helped improve the clarity of the manuscript.
REFERENCES
Adams, A. (2001). Effects of two hurricanes on two assemblages of coral reef fishes: multiple year analysis reverses a false “snapshot” interpretation. Bulletin of Marine Sciences, 69(2), 341–356.
Bell, G. P., Bartholomew, G. A., & Nagy, K. A. (1986). The roles of energetics, water economy, foraging behavior, and geothermal refugia in the distribution of the bat Macrotus californicus. Journal of Comparative Physiology B, 156, 441–450. https://doi.org/10.1007/BF01101107
Boyles, J. G., Cryan, P. M., McCracken, G. F., & Kunz, T. H. (2011). Economic importance of bats in agriculture. Science, 332, 41–42. https://10.1126/science.1201366
Calderón-Acevedo, C., Rodríguez-Durán, A., & Soto-Centeno, A. J. (2021). Effect of land use, habitat suitability, and hurricanes on the population connectivity of an endemic insular bat. Nature Scientific Reports, 11, 9115, https://doi.org/10.1038/s41598-021-88616-7
Furey, N. M. & Racey, P. A. (2016). Conservation ecology of cave bats. In C. C. Voigt and T. Kingston (Eds.), Bats in the Anthropocene: Conservation of bats in a changing world (pp. 463–500). Springer, Cham. https://doi.org/10.1007/978-3-319-25220-9_15
Gamble, D. W., Dogwiler, J. T., & Mylroie, J. (2000). Field assessment of the microclimatology of tropical flank margin caves. Climate Research, 16, 37–50. https://10.3354/cr016037
Gannon, M. R., Kurta, A., Rodríguez-Durán, A., & Willig, M. R. (2005). Bats of Puerto Rico: An island focus and a Caribbean perspective. Texas Tech University Press.
Hayes, J. P., Ober, H. K., & Sherwin, R. E. (2009). Survey and monitoring of bats. In T. H. Kunz & S. Parson, (Eds.), Ecological and Behavioral Methods for the Study of Bats. John Hopkins University Press.
Jones, K. E., Barlow, K. E., Vaughan, N., Rodríguez-Durán, A., & Gannon, M. R. (2001). Short term impacts of extreme environmental disturbance on the bats of Puerto Rico. Animal Conservation, 4, 59–66. https://doi.org/10.1017/S1367943001001068
Kunz, T. H. (1982). Roosting ecology of bats. In T. H. Kunz (Ed.) Ecology of Bats. Plenum Press. https://10.1007/978-1-4613-3421-7_1
Kunz, T. H. & Lumsen, L. F. (2003). Ecology of cavity and foliage roosting bats. In T. H. Kunz & M. B. Fenton (Eds.) Bat Ecology. The University of Chicago Press. http://doi.org/10.5281/zenodo.4655329
Kunz, T. H., Betke, M., Hristov, N. I., & Vonhof, M. J. (2009). Methods for assessing colony size, population size, and relative abundance of bats. In T. H. Kunz & S. Parson (Eds.) Ecological and Behavioral Methods for the Study of Bats. John Hopkins University Press.
Kunz, T. H., de Torrez, E. B., Bauer, D., Lobova, T., & Fleming, T. H. (2011). Ecosystem services provided by bats. Annals of the New York Academy of Sciences, 1223, 1–38. https://doi.org/10.1111/j.1749-6632.2011.06004.x
Kurta, A. & Rodríguez-Durán, A. (2023). Bats of the West Indies: A Natural History and Field Guide. Cornell University Press (In press).
Ladle, R. J., Firmino, J. V. L., Malhado, A. C. M., & Rodríguez-Durán, A. (2012). Unexplored diversity and Conservation potential of Neotropical hot caves. Conservation Biology, 26, 978–982. https://10.1111/j.1523-1739.2012.01936.x
Lugo, A., Miranda Castro, L., Vale, A., López, T., Hernández Prieto, E., García Martinó, A., Puente Rolón, A., Tossas, G., McFarlane, D., Miller, T., Rodríguez, A., Lundberg, J., Thomlinson, J., Colón, J., Schellekens, J., Ramos, O., & Helmer, E. (2001). Puerto Rican Karst – A Vital Resource. (Technical Report WO – 65). United States Department of Agriculture, Forest Service.
Padilla-Rodríguez, E. (2021). Distribución de las cuevas calientes en la zona del carso con prioridad de conservación de Puerto Rico. Perspectivas en Asuntos Ambientales, 9, 55–66. https://documento.uagm.edu/cupey/perspectivas/p_perspectivas_9_2021_55-66.pdf
Rivera-Marchand, B. & Rodríguez-Durán, A. (2001). Preliminary observations on the renal adaptations of bats roosting in hot caves in Puerto Rico. Caribbean Journal of Science, 37, 272–274.
Rodríguez-Durán, A. (1995). Metabolic rates and thermal conductance in four species of Neotropical bats roosting in hot caves. Comparative Biochemistry and Physiology, 110, 347–355. https://doi.org/10.1016/0300-9629(94)00174-R
Rodríguez-Durán, A. (1996). Foraging ecology of the Puerto Rican boa (Epicrates inornatus): Bat predation, carrion feeding, and piracy. Journal of Herpetology, 30, 533–536. https://doi.org/10.2307/1565698
Rodríguez-Durán, A. (1998). Nonrandom aggregations and distribution of cave-dwelling bats in Puerto Rico. Journal of Mammalogy, 79, 141–146. https://doi.org/10.2307/1382848
Rodríguez-Durán, A. (2005). Murciélagos. In J. Joglar, (Ed.) Biodiversidad en Puerto Rico: Vertebrados Terrestres y Ecosistemas. ICPR and Universidad Interamericana de Puerto Rico, San Juan.
Rodríguez-Durán, A. (2009). Bat assemblages in the West Indies: The role of caves. In T. H. Fleming and P. Racey (Eds.) Island Bats: Evolution, Ecology and Conservation. The University of Chicago Press.
Rodríguez-Durán, A. (2020). Roosting ecology, the importance of detailed description. In Theodore H. Fleming, Liliana Davalos, and Marco Mello (Eds.) Phyllostomid Bats, a Unique Mammalian Radiation. The University of Chicago Press. https://doi.org/10.7208/ chicago/9780226696263.003.0018
Rodríguez-Durán, A. & Lewis, A. R. (1987). Patterns of population size, diet, and activity time for a multispecies assemblage of bats at a cave in Puerto Rico. Caribbean Journal of Science, 23, 352–360.
Rodríguez-Durán, A. & Christenson, K. (2012). Breviario sobre los Murciélagos de Puerto Rico, La Española y las Islas Vírgenes. Universidad Interamericana de Puerto Rico y Publicaciones Puertorriqueñas.
Rodríguez-Durán, A. & Kunz, T. H. (2001). Biogeography of West Indian bats: An ecological perspective. In C. A. Woods & F. E. Sergile (Eds.) Biogeography of the west Indies: Patterns and Perspectives. CRC Press, NY.
Silva-Taboada, G. (1979). Los murciélagos de Cuba. Editorial de la Academia de Ciencias de Cuba, Habana.
Citation: Rodríguez-Durán, A., Nieves, A., Avilés Ruiz, Y., Martínez, Y., & Andújar-Morales, K. (2023). Population estimates of bat assemblages from hot caves in Puerto Rico. Novitates Caribaea, (22), 13–24.
https://doi.org/10.33800/nc.vi22.336